Abstract
Learned olfactory-guided navigation is a powerful platform for studying how a brain generates goal-directed behaviors. However, the quantitative changes that occur in sensorimotor transformations and the underlying neural circuit substrates to generate such learning-dependent navigation is still unclear. Here we investigate learned sensorimotor processing for navigation in the nematode Caenorhabditis elegans by measuring and modeling experience-dependent odor and salt chemotaxis. We then explore the neural basis of learned odor navigation through perturbation experiments. We develop a novel statistical model to characterize how the worm employs two behavioral strategies: a biased random walk and weathervaning. We infer weights on these strategies and characterize sensorimotor kernels that govern them by fitting our model to the worm’s time-varying navigation trajectories and precise sensory experiences. After olfactory learning, the fitted odor kernels reflect how appetitive and aversive trained worms up- and down-regulate both strategies, respectively. The model predicts an animal’s past olfactory learning experience with > 90% accuracy given finite observations, outperforming a classical chemotaxis metric. The model trained on natural odors further predicts the animals’ learning-dependent response to optogenetically induced odor perception. Our measurements and model show that behavioral variability is altered by learning—trained worms exhibit less variable navigation than naive ones. Genetically disrupting individual interneuron classes downstream of an odor-sensing neuron reveals that learned navigation strategies are distributed in the network. Together, we present a flexible navigation algorithm that is supported by distributed neural computation in a compact brain.
Author summary
Learning is a feature of species across scales. How does the brain flexibly learn to produce complex behavior? We focus on C. elegans to study how navigation strategies depend on olfactory learning by utilizing precise measurements and a novel model. The fitted model shows that learning alters two known navigation strategies in worms and the model outperforms a classical metric in decoding the animal’s past learning experience. We discover that learned navigation can express itself in a context-dependent manner. In addition, through perturbing individual interneurons, we find that most neurons’ contributions are distributed across strategies and are differentially altered by learning. We expect these flexible behavioral algorithms and neural computations to be generalized to other species and behavior.
Citation: Chen KS, Sharma AK, Pillow JW, Leifer AM (2025) Navigation strategies in Caenorhabditis elegans are differentially altered by learning. PLoS Biol 23(3):
e3003005.
https://doi.org/10.1371/journal.pbio.3003005
Editor: Piali Sengupta, Brandeis University, UNITED STATES OF AMERICA
Received: October 22, 2024; Accepted: January 7, 2025; Published: March 21, 2025
Copyright: © 2025 Chen et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Data to generate all figures in this work are available on the figshare website: doi: 10.6084/m9.figshare.24764403. The numerical data for all replicates underlying the figure panels with summery statistics are in the S1_Data.xlsx file in figshare. The Code used for analysis and to generate figure panels are archived at doi: 10.5281/zenodo.14542447.
Funding: This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke (https://www.ninds.nih.gov/) under New Innovator award (DP2-NS116768 to AML), the Simons Foundation (https://www.simonsfoundation.org/) under award SCGB (543003 to AML and AWD543027 to JWP), the National Science Foundation (https://www.nsf.gov/) through NSF CAREER Award (NSF PHY-1748958 to AML), NIH BRAIN initiative (https://braininitiative.nih.gov/) (R01EB026946 to JWP), U19 NIH-NINDS BRAIN Initiative Award (U19NS123716 to JWP), and through the Center for the Physics of Biological Function (PHY-1734030). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Learning is a fundamental property of neural systems that allows an animal to flexibly alter behavior based on experience. Learned olfactory navigation provides an ideal framework for studying learning because it is a naturalistic behavior, common to species across scales [1,2], ethologically relevant for seeking food [3,4] and avoiding pathogens [5,6], and can be rapidly learned in very few trials [4,7,8]. Careful characterization of learned navigation behavior in a naturalistic context can shed light on the flexible computation performed by the brain [9].
To study how animals flexibly alter their olfactory navigation upon learning, we focus on the nematode worm C. elegans. This worm has a compact nervous system [10], well-characterized olfactory neural circuits [11–15] and navigation behavior that has been studied in detail [11,16]. Worms learn to associate an odor with the presence or absence of food and then will either navigate towards higher concentrations of the odor or will ignore the odor, respectively [3,17]. When learning to associate the odor butanone to a food source, it is unknown how the worm’s navigation strategies are altered by learning. We aim to quantitatively characterize the worm’s navigation strategy and the underlying sensory transformations in order to constrain neural mechanisms of learned navigation.
The worm navigates in sensory environments mainly through two strategies: klinotaxis and klinokinesis [16,18,19]. Klinotaxis is a process in which the worm continuously modulates its heading to align with the local gradient in space, also known as “weathervaning” [18]. Klinokinesis is a biased random walk [20,21] in which the worm produces sharp turns called “pirouettes” with a probability that depends on the animal’s estimate of the local gradient of a sensory cue [16,20,22]. In both cases the animal estimates information about the local gradient by comparing measurements of the stimuli across time and space: during klinokinesis the worm samples odor concentration at different points along its locomotory trajectory, while for klinotaxis it samples concentrations between shorter side-to-side swings of its head. Both strategies contribute to sensory-guided navigation in landscapes of temperature [19], salt [18,23,24], and certain odors [11,18,25,26].
Experience-dependent changes to these behavioral strategies have been characterized in some sensory modalities, such as in response to salt [18,23,24], temperature [19,27,28], and bacterial food [6,29,30], but it remains less known for learning in response to odorants, such as butanone. For instance, it is not known whether worms adopt entirely new navigation strategies after odor learning, or whether they modulate existing ones. It is plausible that olfactory learning could influence behavioral strategies in a manner similar to learning in the context of salt and temperature, but this hypothesis still requires empirical validation.
Quantitative analysis for airborne odor-guided navigation has been limited, in part because of experimental challenges in measuring detailed information about the odor concentration experienced by the animal. While past work estimated odor cues indirectly or with models [4,18], precise concentration measurements are required to characterize sensorimotor transformation in the sensory environment. Recently, however, new experimental methods to control and monitor the odor landscape experienced by small animals, such as the worm, [31–33], now make it possible to empirically constrain quantitative models of odor-guided navigation, as we pursue here.
Worms are intrinsically attracted to butanone, a volatile organic compound found in bacterial food in the worm’s natural habitat [34]. When worms are exposed to butanone paired with food (“appetitive training”), they increase their attraction and are more likely to navigate towards higher butanone concentrations [3,12,17,35]. In contrast, when butanone is paired with starvation (“aversive training”), worms decrease their tendency to climb up butanone gradients in comparison to worms without exposure to butanone (“naive”) [3,35]. The animal’s neural and behavioral responses to butanone both change upon learning [12,35]. Sensory neuron AWCON responds to butanone [3,35], as well as others [12,36]. However, the involvement of downstream interneurons in butanone learning and learned navigation are still unclear.
In this study we seek to answer: (1) How are the worm’s navigation strategies altered by olfactory learning? (2) How are sensorimotor transformations altered by learning and how does this vary across a population? (3) What neural substrates may be involved? To answer these questions, we combine precise experimental measurements using our recently developed continuous odor monitoring assay [31] and a novel statistical model to rigorously characterize how butanone associative learning alters odor navigation strategies in worms.
Our measurements and model reveals that the animal’s biased random walk is differentially altered by butanone learning and that its weathervaning strategy is down-regulated upon aversive learning. Our approach yields interpretable model parameters that better decode training conditions compared to chemotaxis index, and also predict response to optogenetic perturbation in the sensory neuron AWCON. We discover that naive worms have higher behavioral variability and demonstrate context-dependent behavior. And we provide insights into the role of specific interneurons.
Fig 1. Differentially modulated olfactory learning in C. elegans. (a) Protocol for butanone associative training in worms. (b) After exposure to different training regimens in (a), worms’ olfactory navigation is measured in a controlled odor environment. (c) Example trajectory after three different training conditions. (d) Summary statistics of learning across three conditions. Error bar shows standard error of mean across 9-13 plates for each condition. For each index, all pairs of training conditions (appetitive-naive, naive-aversive, and appetitive-aversive) show statistically significant differences (t-test, p 10.6084/m9.figshare.24764403.
Results
Learning differentially alters olfactory navigation
We developed a protocol to train worms to associate butanone with either food (appetitive training) or starvation (aversive training) (Fig 1a). Our protocol was similar to previously reported training regimens [12,17,35] except that ours exposes the animal to multiple rounds of odor paired with starvation instead of only one, which we found increases consistency in learning (Fig A in S1 File). After training, we recorded the movement of populations of worms as they crawled in a defined odor landscape that used metal-oxide sensors to continuously monitor the odor concentration along the boundary [31] (Fig 1b; Fig B in S1 File). We recorded hundreds of locomotory trajectories per plate in this odor environment after different training conditions, for up to 13 plates per training condition. Animal’s locomotory trajectories were qualitatively different depending upon learning, with appetitive trained animals traveling up-gradient more often than naive animals, and aversive-trained animals traveling up-gradient least of all, broadly consistent with prior reports [3,12,35] (Fig 1c). We quantify the performance of traveling up the gradient using a “chemotaxis index”,
To explore whether worm navigation superficially resembles a biased random walk, we calculated a “run index”,
While the metrics above suggest hypotheses about how navigational strategies change due to learning, they provide little information about the dynamics of navigation, nor the sensorimotor transformations that govern these dynamics. Specifically, the metrics use only binary information about whether at the end of their track the animal traveled up- or down-gradient, and it ignore details of the sensory landscape in between and omits dynamic information like the odor concentration the animal experienced over time. The indices also provide no information about the behavioral variability across the population. To overcome these limitations, we sought a statistical model that captures temporal information, behavioral noise, and explicitly predicts how the animal changes its movement in response to sensory stimuli.
Odor-dependent mixture model of olfactory navigation
To characterize how olfactory sensory inputs are transformed to behavior under different training conditions, we developed a dynamic Pirouette and Weathervaning (dPAW) statistical model of worm olfactory navigation. The dPAW model consists of a mixture of two navigation strategies: a pirouette behavior consisting of an abrupt change in heading angle (a turn) and weathervaning which instead continuously modulates heading angle. The dPAW model describes how the worm implements and balances these two behavioral strategies depending on time-varying sensory inputs (Fig 2a). The dPAW model is an extension to a classic biased random walk, where the run intervals are replaced with weathervaning behavior. This framework explicitly models these strategies and is fit to detailed measurements of movement and odor-experience. This is to our knowledge the first statistical model that explicitly captures the detailed changes to C. elegans navigation strategies upon butanone learning.
The model worm samples its heading change dθ at each time step from one of two distributions: either a “weathervaning distribution”
The worm’s decision to initiate a pirouette or to continue to weathervane on each time step is modeled with a Bernoulli generalized linear model (GLM) that takes the filtered history of the odor concentration
where
is the mixing probability over the two distributions, with parameters m and M for the minimum and maximum probability of a pirouette on a single time bin, and
where U is uniform in the circular heading and f is a von Mises distribution with mean and precision parameter κ. In the pirouette distribution, scalar α ∈ [ 0 , 1 ] is the weight on the uniform distribution and
We fit dPAW to measurements of animal movement in the odor arena, including the time varying headings
Model captures navigation altered by olfactory learning
To characterize how olfactory learning alters navigation strategies, we fit the dPAW model to trajectories measured in the butanone odor environment after different training conditions. We first confirmed that the model captures key aspects of the animal’s navigation. We confirmed that the fitted model’s estimate of pirouette frequency matches that measured empirically [16] (Fig Db in S1 File). We also used the model to simulate chemotaxis behavior in the odor environment and confirmed that model-generated trajectories have a chemotaxis index that agrees with measurement and is similarly differentially modulated by training conditions (Fig 2b). Model-generated trajectories also appear visually similar to experimental observations (Fig 2c). And in further agreement, trajectories simulated from the model recapitulate sensory and behavioral statistics measured in experiments, including the measured distribution of the worm’s heading angle, pirouette rate, experienced perpendicular odor concentration difference, and tangential odor concentration (Fig D and Fig E in S1 File).
Agreement between model and measurement was not due to chance. The dPAW model on average captures 0 . 3 to 0 . 6 bits/s more information about navigation behavior than a null model that lacks any olfactory sensing mechanisms (Fig 4c). For comparison to additional null models see Fig E in S1 File.
Sensorimotor kernels change upon learning
We hypothesize that the sensorimotor temporal kernels,
The kernels corresponding to pirouette decisions,
Collectively, we show that C. elegans alter their chemotaxis upon learning by changing the kernels that govern their sensorimotor response.
Model also captures bidirectional salt chemotaxis
To gain confidence in dPAW’s ability to extract meaningful sensorimotor kernels and parameters, we also challenged dPAW to capture changes to sensorimotor transformations in a different context, namely context-dependent changes to salt chemotaxis (Fig 3a– 3c). Navigation strategies in a salt environment have been characterized previously—worms navigate towards the concentration they were raised at by employing both biased random walk and weathervaning strategies [18,23] (Fig 3d). We repeated a series of experiments previously reported in [23]: animals raised at 50 mM salt concentration were evaluated on linear salt gradients of different concentration regimes (0 to 50 mM, and 50 mM to 100 mM). In each case dPAW was fit to measurements of the animal’s locomotory trajectories. As expected, the dPAW model captured positive and negative salt chemotaxis, depending on the environment the animals were tested on. (The paradigm commonly used for salt chemotaxis differs from that of butanone—for salt the animal’s experience stays the same but the arena varies, while for butanone the experience varies but the arena stays the same.) Reassuredly, simulations from dPAW fitted to salt chemotaxis trajectories nicely agreed with the observed positive and negative chemotaxis indices (Fig 3g and 3h). Sensory kernels had not previously been reported for salt chemotaxis, but one would expect their kernels’ sign to switch depending on whether the worm was in a higher or lower salt concentration landscape. Indeed, dPAW’s inferred salt sensorimotor kernels for both biased random walk (
Decision function governing pirouettes changes upon odor learning
To understand how the animal alters its preference for continuing to weathervane versus interrupting weathervaning with pirouettes during odor learning, we compared the pirouette decision function in Eq 2 inferred from our measurements before and after learning.
We found that learning alters the input-output statistics governing the initiation of a pirouette. This is clear from inspecting the distribution of the filtered signal (Fig 4a, bottom, related to odor concentration and past behavior) that serves as input to the decision function. Appetitive training and aversive training push the tail of the filtered signal probability distribution in opposite directions with respect to naive condition, which changes the statistics of pirouettes. This could reflect either changes to the kernels, or changes to the environment that those animals prefer to explore.
Aversive-trained worms have higher baseline turning rate m than appetitive trained worms, as seen in the output probability (Fig 4a, right), which is the result of passing the filtered odor signal (Fig 4a, bottom) through a nonlinear decision function (Eq 2 and Fig 4a, top). Both aversive- and appetitive-trained worms have higher maximum pirouette rate M compared to naive worms, reflecting a change to the decision function itself.
It is interesting to note that appetitive (but not aversive) trained animals spend more time with their pirouette probabilities in the most sensitive range of the sigmoid (Fig 4a right, inset), possibly indicating a more efficient strategy for chemotaxis. Capturing these details is one of the ways that dPAW is able to better detect changes in learning.
Model outperforms other metrics at decoding learned experience
The changes to sensorimotor processing detected by the dPAW model all provide information about the animal’s past experience. We therefore wondered whether the model could accurately predict the training (aversive, appetitive or naive) that a population of worms had experienced. To test this we inspected dPAW models fit to measured trajectories from each training conditions γ ∈ Halo corresponding to fitting parameters
The inferred kernels and decision function within
Some training conditions are more challenging for dPAW to decode than others. Naive worms show the lowest predictive performance with finite data when decoding is performed for each training condition separately (Fig 5b). This is consistent with our estimate of lower information exhibited by the trajectories of naive worms than trained worms (Fig 4c). Practically, this means more measurements are required to capture navigation behavior in naive worms than in trained worms.
The same computations govern natural and optogenetic odor stimuli
We wondered whether the same underlying computations that governed the animal’s response to natural odor stimuli also govern its response to optogenetic-induced sensory stimuli. Optogenetics stimuli are not bound by the same natural statistics that the worm encounters when exploring a physical odor arena. For example, worms in our arena always experience temporally correlated and slowly varying odor responses. This introduces potentially confounding temporal correlations that can be avoided by using optogenetic stimuli [35,37–39]. We therefore investigated the animal’s behavioral response to optogenetic induced odor sensation after learning and adapted our model to incorporate both forms of stimuli.
The animal’s behavioral response to optogenetic stimulation was differentially altered by learning (Fig 6; Fig H and Fig I in S1 File). We measured the animal’s absolute change in heading angle | dθ | in response to optogenetic stimulation of neuron AWCON expressing ChR2 (Fig 6b). AWCON is a butanone sensitive neuron known to play an important role in olfactory learning [12,17,35,39]. We measured behavior response to optogenetic stimuli two ways: we delivered pulses of optogenetic stimuli to animals experiencing odor in the arena (Fig 6; Fig H in S1 File), and we delivered time-varying intensity white noise optogenetic stimuli to animals off odor (Fig I in S1 File). In both cases (Fig 6e; Fig I in S1 File) the animal’s turning behavior was more tightly coupled to optogenetic stimuli for appetitive trained animals than naive worms, and less so for aversive trained animals. A change in behavior response is consistent with prior reports [35,39], but note that here we make a more stringent comparison by comparing both appetitive and aversive trained animals against the same naive control condition.
We extended our model so that light intensity contributes to the pirouette probability during navigation:
where kernels
Across different training conditions, the optical kernel is close to the mirror image of the odor kernel, most strikingly demonstrated in the naive and aversive conditions (Fig 6e). Therefore the sensorimotor computation inferred from freely moving animals is predictive of the response to external perturbations. The inversion is expected and can partly be explained by the biophysics of the AWCON neuron: it hyperpolarizes when odor is present and depolarizes when odor is removed. This gives us confidence that our findings about sensorimotor processing derived from natural odor stimuli should be relevant to the larger literature based on more artificial stimulation. We therefore will leverage optogenetics to probe behavioral variability during odor navigation.
Learning modulates behavioral variability in response to sensory perturbation
We sought to characterize the variability in learned odor-guided navigation, because variability is a known feature of sensory processing in worms [40]. We observe variability across collections of trajectories in learned navigation. For example, the kernel
Surprisingly, we discovered that animals respond to stimuli differently depending on whether they are traveling up or down an odor gradient and that this contributes to variability (Fig 6c and 6d). Naive worms respond more strongly to optogenetic impulses delivered when traveling up an odor gradient, than when traveling down the gradient (Fig 6c). Appetitive trained worms, by contrast, respond consistently to optogenetic impulses regardless of their direction of travel along the odor gradient. Aversive trained worms show weak response to optogenetic impulse regardless of the gradient context. This indicates that learning alters behavioral variability, and that a response to stimuli can be context-dependent along the navigation trajectory.
Downstream interneurons differentially contribute to learned chemotaxis
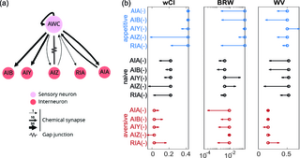
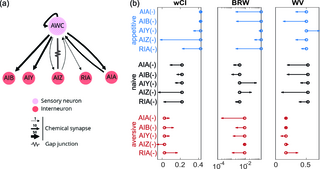
We investigated interneurons that may be involved in implementing learned changes to navigation strategy. We focused on interneurons downstream of the odor-sensing neuron AWCON because optogenetically induced activity in AWCON recapitulates aspects of learned navigation (Fig 6b) and AWCON is known to play an important role in odor sensing [11], navigation [41–43], and butanone learning [3,35]. We selected five interneurons subtypes with direct synaptic inputs (chemical or electrical connections) from AWCON [10,13]: AIA, AIB, AIZ, AIY, and RIA, several of which are known to be involved in navigation [18,19,23,35,42,44]. For each neuron subtype we compared learned odor-guided navigation in wild type animals to that of transgenic strains for which the neuron subtype was genetically ablated (via miniSOG, Methods Table 1) or down-regulated (via expressing activated potassium channel), as described in methods, Fig 7.
Ablating or down-regulating the downstream interneurons had wide-ranging and statistically significant effects on chemotaxis performance and inferred model parameter, including after learning (Fig 7b; Fig J and Fig K in S1 File). Here we characterize chemotaxis performance with a weighted chemotaxis index (wCI) that differs from the chemotaxis index in Fig 1d by more heavily weighting trajectories that experience a large change in odor concentration and de-emphasizing tracks that experience little odor change (described in the methods). To capture changes in inferred model parameters we report a stimuli-normalized magnitude of the kernels
Ablation of neuron subtype AIZ was most severe and eliminated gradient climbing behavior across all three conditions (wCI close to zero), consistent with its reported role in the context of salt chemotaxis [18]. In general, though, neuron subtypes contributed differently to chemotaxis performance, learning, or had different contributions to the inferred kernels corresponding to each navigation strategy. For instance, AIA and AIB defective animals showed little difference between naive and aversive training conditions, but did increase chemotaxis performance after appetitive training. In other words, AIA and AIB are less involved in appetitive behavior but important to maintain naive and aversive behavior. The AIY defective animals show an interesting increase in their use of weathervaning after appetitive training and an increase in their use of biased random walk in naive conditions. This suggests that the recruitment of AIY for different strategies may be learning-dependent. The RIA defective animals have similar chemotaxis performance upon appetitive and aversive learning, suggesting that RIA is involved in fine-tuning the animal’s heading angle in response to learning. Interestingly, RIA defect results in much lower gradient climbing performance for naive animals. This suggests that, in addition to its role in learning, RIA may also be important for sensory adaptation, including of naive animals, as they perform odor navigation.
In general, we do not observe a simple one-to-one mapping of neuron sub-populations to chemotaxis strategies. This suggests that even though weathervaning and biased random walk strategies are mathematically and behaviorally distinct, they do not segregate cleanly into different neuron types. Instead the neural basis for learning-dependent weathervaning and biased random walks are both distributed across the neural population.
In our investigation, we apply the same model to multiple transgenic strains. This has not commonly been done in the past because it can be challenging to find a model that is general enough to accommodate differences across strains. A potential confound can arise if the transgenic strains’ behavior differs from wild-type in such a way as to be poorly captured by the model. And, indeed, we do observe some differences in the behavior of different strains, for example their speed distributions differ (Fig Jb in S1 File). Reassuredly, several strands of evidence suggest that the differences dPAW infers in navigation across transgenic strains in Fig 7 reflect differences in navigation strategies, and can not be trivially explained by model mismatch. First, dPAW’s cross-validated fit for transgenic strains is not dramatically different from those of wild-type worms (Fig La in S1 File), suggesting that the model is doing a decent job capturing transgenic strains’ behavior. Second, an analysis of heading distributions further shows reasonable agreement between model and measurements (Fig Lb in S1 File). Finally, the model’s microscopic description of transgenic strains’ behavior does a reasonable job recapitulating the worms’ observed macroscopic chemotaxis index (Fig M in S1 File). When considering all strains across all learning conditions, the model’s predicted chemotaxis index correlated to the measured chemotaxis index (
Discussion
We combined precise experimental measurements and a novel statistical model, dPAW, to characterize learned olfactory navigation in worms. The results show that (1) navigation strategies are differentially altered by learning, (2) the dPAW model decodes the animal’s past training conditions based on the observed navigation behavior and outperforms a classic chemotaxis metric, (3) behavior is more variable in naive worms and this variability can in part be explained by a context-dependent response to odor, and (4) interneurons downstream from AWCON contribute to learned navigation strategies in a distributed manner.
To reach these conclusions, two experimental advances were critical. The first was the measurement of navigation in a precisely defined sensory environment enabled by our recently developed odor delivery system [31]. Previous studies had analyzed navigation based on the proximity to a droplet source [3,12,35], but in those experiments the precise odor concentration experienced by the animal was typically not known. With the odor delivery system, we obtained the odor concentration experienced by the animal and therefore were able to fit more comprehensive models of sensorimotor processing.
The second methodological advance is the development of a more robust training protocol to probe differential learning effects (aversive and appetitive) that shows clearer effects when tested in the same odor concentration range. Prior investigations into butanone learning probed different associative learning (appetitive vs. naive, or aversive vs. naive) in different concentration regimes [3,17,35,45], possibly because the behavior effects of aversive learning are known to be more pronounced at lower odor concentrations of butanone while appetitive is known to be more pronounced at higher concentrations of butanone (see for example Fig 1- figure supplement 2 of [12] and results in [46]). But here we sought to directly compare detailed properties of learning, such as kernels, in the same sensory environment. To do so, we changed the training protocol (more repetitions during aversive than appetitive) in order to achieve a greater difference in learning outcomes. This produced large differential training-dependent changes to chemotaxis that were visible even in the same sensory environment (Fig 1).
The dPAW model fitted to measurements provides new insight into how the animal responds to time varying sensory stimuli. In particular, we find that learning alters the temporal kernels for odor input. For instance, appetitive training sharpens the tangential concentration kernel (Fig 2). By contrast, classical approaches have missed this important change because they implicitly have static sensing kernels and a priori fix the time window to calculated gradients [16,18,19,23].
Our measurement and model reveals that learning alters not only the sensing mechanism but also the statistics of behavioral noise (Fig 4), which is consistent with recent work showing that starvation and neuromodulation can dramatically alter the statistics of behavior [27,47]. Our finding was possible only because dPAW explicitly includes parameters that control the noise level of the behavioral output, which is another advantage over past work which often excludes noise and relies on predetermined parameters [16,18,48]. Future work is needed to pinpoint the source of behavioral stochasticity, such as noise in the sensory or motor circuits, and its potential functional roles in navigation and exploration [40,49].
A strength of our approach is that it allows us to learn properties of sensorimotor kernels from either artificial optogenetic stimulation or natural odor stimuli. Past work, by contrast, allowed either optogenetic stimulation [35,39] or odor stimuli [33,41], but not both. Optogenetic stimulation has advantages because it can be finely manipulated to deliver rich informative stimuli, for example white noise stimuli to study sensory encoding [50,51]. But there are challenges to connect optogenetic stimuli to natural stimuli experienced during navigation. Our work directly shows that information from one approach is compatible to the other. We applied statistical inference directly to navigational trajectories in the presence of odor and qualitatively recover similar sensorimotor transformations to those we inferred using optogenetic perturbation (Fig 6). This is to our knowledge the first direct comparison between sensorimotor computation during navigation and response upon external perturbation.
An important conclusion from optogenetic perturbations is that the worm’s response variability is altered by learning. We found that naive worms are more variable and that learning reduces variability in behavioral strategies across the population of worms. Surprisingly, we further found that the variation across optogenetic responses in naive worms can in part be explained by the context of that worm’s odor environment, up or down the gradient (Fig 6). Naive worms that travel up-gradient pay more attention to both odor and optogenetic stimuli (Fig 6c; Fig H in S1 File) compared to those that go down-gradient. This is broadly consistent with prior literature describing how the result of associative learning can be heterogeneous across animals or non-stationary in time [52–54]. Measurements in response to optogenetically perturbing AWCON suggest that the source of such context-dependent behavior might be within or downstream of AWCON. Future work is needed to address the source of such variability in the nervous system, as well as its possible functional role for navigation and searching behavior [1,40].
Of the interneurons we investigated, all five classes contribute to both learned navigation strategies (Fig 7). Importantly, many connections from AWCON to the first layer of interneurons are shared with salt sensing neurons ASER/L and thermal sensing neuron AFD [18,19,23]. Some past work hypothesize that experience-dependent changes are localized in specific neurons—AIB has been shown to play a role in context-dependent thermal navigation [19]. But other work presents evidence that learning effects can be distributed across many neurons [12,23]. For instance, AIB, AIY, and AIZ all seem to be involved in learned salt chemotaxis strategies [23]. Our findings agree with these results in showing that all three interneurons have non-zero and learning-dependent contributions to both behavioral strategies.
We investigated a selection of key interneurons, but we cannot rule out the role of other neurons that we did not investigate, including conceivably other sensory neurons or modulatory signals [12,35,55,56]. We also studied single neuron-type manipulations one at a time. Future work could explore combinatorial effects by perturbing multiple neuron types at once [6,23]. Finally, we relied on chronic molecular perturbations which risk introducing off-target or compensatory effects to wiring or other neurons during development. Future exploration of neural mechanisms can include more transient perturbations or could be pursued in combination with neural imaging methods.
One puzzling finding is that we sometimes notice changed behavioral strategies but unchanged chemotaxis index. For example, appetitive trained worms with defects in AIB have dramatic reductions in both weathervaning and biased random walk strategies but still show reasonable chemotaxis performance. This may be due to larger model mismatch in transgenic worms with strategies deviating from dPAW. Large variability and less consistent behavioral strategies were also observed in earlier ablation studies [18]. We explore this further in the supplement (Text A in S1 File).
In this work we utilize a controlled odor environment and an innovative model to characterize learned odor navigation in worms. The combined approach of precisely delivered sensory stimuli, behavior quantification and navigational modeling continues to be powerful [9,57,58] and can be generalized to other sensory modalities and species to study adaptive sensory navigation. By identifying the specific features of behavior that are altered by learning, our investigation lays the groundwork for followup neural imaging studies and will guide our search for neural representations of learning [59,60].
Materials and methods
Worm strains and preparation
All worms were maintained at 20 C on nematode growth medium (NGM) agar plates seeded with E. coli (OP50). We used N2 bristol as wild type worms. A detailed strain list is provided in Table 1. Strain AML105 used for optogenetics experiments was integrated using strain CX14418 from [35] employing UV irradiation and was outcrossed six times before testing. AIB(-) strain is from [31] and AIA(-), AIY(-), AIZ(-) and RIA(-) strains are from [? ]. All worm strains used in this work are shown in Table 1.
Before chemotaxis experiments, we bleached and centrifuged batches of worms to synchronize the next generation as described in [61]. L1 synchronized worms were plated to seeded NGM plates on the next day. Experiments were conducted 3 days after seeding, which corresponds to synchronized 1-day-old adult worms. For optogenetic strains, L1 stage worms were plated onto 9 cm NGM agar plates seeded with 1ml OP50 food with 10 μl all-trans retinal (from 100 mM stock). For interneuron perturbation, miniSOG strains were treated with square wave pulses of 2.16 mW/mm2 450 nm blue light at 1 Hz for 30 minutes at L1 stage and then allowed to recover before testing. The combination of strains and perturbations used in this work are detailed in Table 2.
Olfactory learning protocol
The olfactory learning protocol was adapted, with modifications, from previously described protocols [3,12,17,35]. Synchronized young adult worms were removed from food and washed three times with S. Basal solution [11]. For appetitive training, worms were suspended in 10 ml of S. Basal solution on a shaker to starve for 1 hour. After starvation, worms were placed on a 9 cm NGM agar plate with 1 ml of OP50 and 12 μl of pure butanone (2-Butanone, +99%, Extra Pure, Thermo Scientific) dropped on the interior of the lid and sealed with Parafilm. To fixate butanone droplets on the lid, we placed 3 agar plugs on the lid and dropped 4 μl of butanone onto each plug. To conduct aversive training, worms were suspended in 10 ml of S. Basal with 1 μl butanone added. The tube was sealed and placed on the shaker for 1 hour. We found that aversive training was most robust with repetition (Fig A in S1 File), so we interleaved the session by plating the worms back on food for 30 minutes, then repeating the training for three times (Fig 1a). Worms were washed three times with S. Basal solution and centrifuged between each transfer during the training protocol. For naive condition, worms were directly removed from food and washed for three times before testing.
Odor delivery system and chemotaxis experiments
We used the odor flow delivery system and experimental protocols developed in [31] to measure chemotaxis trajectories after different training conditions. In short, this system incorporates controlled odorized airflow and continuously measures odor concentration along the boundary of an agar plate during animal experiments. As in [31], we calibrated the full array of metal-oxide based sensors with a downstream photo-ionization detector (Fig Ba in S1 File) to characterize the steady-state spatial profile (Fig Bb in S1 File). During animal experiments, we swapped out sensors in the middle of the arena to place in worms on an agar plate and continued to measure the boundary condition to confirm that the odor landscape is controlled and stable across the arena (Fig Bc in S1 File), as in [31] .
For butanone chemotaxis experiments, we used 1.6 % agar with salt content matching S. Basal solution in a 10 cm square plate lid. We used 11 mM butanone dissolved in water as the odor reservoir and provided moisturized clean air as the background flow. The background airflow was 400 ml/min and the butanone odor flow was 33–36 ml/min. After the pre-equilibration protocol [31] that brings the agar plate to steady-state in the odor environment, 50–100 worms were placed in the middle of agar plate and dried with kimwipes. Each chemotaxis sessions was recorded for 30 minutes.
Behavioral imaging and optical setup
Worm behavior imaging was performed as described in [31]. Briefly, a CMOS camera measured worm behavior at 14 Hz. Worms were illuminated with 850 nm light. Images were captured with custom written Labview program and analyzed with Matlab scripts. An important difference from [31] is that here we added the ability to deliver optogenetic stimulation. Three 455 nm LEDs (M455L4, Thorlabs) were fixed on top of the flow chamber to deliver stimulation. The intensity was calibrated in the field of view with a photometer, such that 85 μW/mm2 intensity light was uniformly delivered. Each pulse lasts for 5 seconds and were delivered every 30 seconds. Here we analyzed 1220 to 3060 pulses delivered to worms treated with retinal in each training condition.
Chemotaxis assay in linear salt gradients
We conducted salt chemotaxis experiments following established methods [23]. We constructed linear salt gradient in the same 10 cm square plate lids used for odor delivery. Worms grown on standard NGM plates with 50 mM salt concentration were washed off food with M9 solution, then placed in the middle of either the 0–50 mM or 50–100 mM linear gradient. We then imaged behavior in the same odor flow chamber and imaging setup without applying airflow.
Behavioral analysis and dPAW inference
We tracked the location of the worm’s centroid and fit a centerline to its posture as described in [31]. We removed trajectories that are shorter than 1 minute or have displacement less than 3 mm across the recordings. In addition, trajectories that started above 70% of the maximum odor concentration were also removed to prevent double-counting worms that may have already started or traveled up-gradient. This results in 270 to 1,140 animal hours of chemotaxis trajectory per training condition for model fitting.
We characterized chemotaxis performance and strategies with indices in Fig 1d and Fig 3b. The chemotaxis index is
To analyze the time series of navigation, for each processed navigation trajectory, we computed the displacement vectors every 5 time bins (5/14 seconds) and computed the angle between consecutive vectors to obtain
We fit dPAW to the ensemble of trajectories by maximizing the log-likelihood:
where N is the number of trajectories, T is the time steps, and λ is the regularization term. To impose smoothness on kernels,
Uncertainty about the inferred parameters are characterized by numerically computing the Hessian of the log-likelihood function around the maximum likelihood estimation. For model-based decoding, we perform 7-fold cross-validation with all measured trajectories. To analyze effects with finite data, we subsample 10 ensembles of trajectories to test for performance in Fig 5.
To validate the accuracy of maximum likelihood inference for dPAW model parameters, we simulated behavioral time series from dPAW with a fixed set of ground-truth parameters. The model generated simulated trajectories using Gaussian random white noise for concentration time series
To generate chemotaxis trajectories from inferred parameters shown in Fig 2, we conducted agent-based simulation by measuring concentration in the same odor landscape and drawing angular change
where
For information rate (Fig 4) and model comparison (Fig 5), we constructed null models to compare with dPAW. The random walk model has similar statistical structure for behavior but is independent of odor concentration:
Note that in this null model, the turning probability is time-independent, so β does not have a time subscript t. The pirouette behavior has the similar sharp angle as the full model, but now in the null model the weathervaning is changed to “runs” that have zero-mean and do not take perpendicular concentration change into account. This model was fitted to the same ensemble of trajectories, and the log-likelihood difference between the full dPAW and this null model was normalized by log ( 2 ) per time to compute the bit rate. We also used this model to compute behavior-only model predictions in Fig 5. The chemotaxis model is formulated with a binomial distribution with expected fraction of tracks going up-gradient
Statistical analysis for chemotaxis in transgenic worms
We computed the concentration weighted chemotaxis index (wCI) as follows,:
To conduct statistical tests between indices in Fig 7, we re-sampled chemotaxis trajectories in each condition (Fig K in S1 File). For the weighted chemotaxis index, we sampled 50 tracks for 20 times to compute the standard deviation from mean. For the biased random walk strategy, we computed
Measuring the behavior-triggered average with optogenetics
For behavior triggered averages (Fig I in S1 File) we delivered time varying LED light stimuli drawn from N ( 30 , 30 ) (μW/mm2) at 14Hz with 0.5s correlation time and bounded between 0-60 μW/mm2. This serves as a white noise stimulus. We computed the behavior triggered average (BTA) for reversals following methods applied to touch sensation in worms [61].
Acknowledgments
We thank the Leifer lab and Pillow lab for helpful discussions. We also like to thank Prof. Cori Bargmann for sharing worm strain CX14418 and Prof. Ikue Mori for sharing worm strains IK2962, IK3240, IK3241 and IK3289.
References
- 1.
Baker KL, Dickinson M, Findley TM, Gire DH, Louis M, Suver MP, et al. Algorithms for olfactory search across species. J Neurosci 2018;38(44):9383–9. pmid:30381430 - 2.
Porter J, Craven B, Khan RM, Chang SJ, Kang I, Judkewitz B, et al. Mechanisms of scent-tracking in humans. Nat Neurosci. 2007;10(1):27–29. - 3.
Torayama I, Ishihara T, Katsura I. Caenorhabditis elegans integrates the signals of butanone and food to enhance chemotaxis to butanone. J Neurosci 2007;27(4):741–50. pmid:17251413 - 4.
Gire DH, Kapoor V, Arrighi-Allisan A, Seminara A, Murthy VN. Mice develop efficient strategies for foraging and navigation using complex natural stimuli. Curr Biol 2016;26(10):1261–73. pmid:27112299 - 5.
Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005;438(7065):179–84. pmid:16281027 - 6.
Ha H, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, et al. Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 2010;68(6):1173–86. pmid:21172617 - 7.
Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D. Neuroscience needs behavior: correcting a reductionist bias. Neuron 2017;93(3):480–90. pmid:28182904 - 8.
Meister M. Learning, fast and slow. Curr Opin Neurobiol. 2022;75:102555. pmid:35617751 - 9.
Clark DA, Freifeld L, Clandinin TR. Mapping and cracking sensorimotor circuits in genetic model organisms. Neuron 2013;78(4):583–95. pmid:23719159 - 10.
White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 1986;314(1165):1–340. pmid:22462104 - 11.
Bargmann CI. Chemosensation in C. elegans. WormBook. 2006; p. 1–29. - 12.
Pritz C, Itskovits E, Bokman E, Ruach R, Gritsenko V, Nelken T, et al. Principles for coding associative memories in a compact neural network. Elife. 2023;12:e74434. pmid:37140557 - 13.
Cook SJ, Jarrell TA, Brittin CA, Wang Y, Bloniarz AE, Yakovlev MA, et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature 2019;571(7763):63–71. pmid:31270481 - 14.
Dusenbery DB, Sheridan RE, Russell RL. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics 1975;80(2):297–309. pmid:1132687 - 15.
Croll NA. Sensory mechanisms in nematodes. Annu Rev Phytopathol 1977;15(1):75–89. - 16.
Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci 1999;19(21):9557–69. pmid:10531458 - 17.
C. elegans positive butanone learning, short-term, and long-term associative memory assays. J Vis Exp. 2011;49. - 18.
Iino Y, Yoshida K. Parallel use of two behavioral mechanisms for chemotaxis in Caenorhabditis elegans. J Neurosci 2009;29(17):5370–80. pmid:19403805 - 19.
Ikeda M, Nakano S, Giles A, Xu L, Costa W, Gottschalk A, et al. Context-dependent operation of neural circuits underlies a navigation behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2020;117(11):6178–88. - 20.
Macnab RM, Koshland DE Jr. The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A 1972;69(9):2509–12. pmid:4560688 - 21.
Keller EF, Segel LA. Model for chemotaxis. J Theor Biol 1971;30(2):225–34. pmid:4926701 - 22.
Berg HC. Random walks in biology. Princeton University Press; 1993. - 23.
Luo L, Wen Q, Ren J, Hendricks M, Gershow M, Qin Y, et al. Dynamic encoding of perception, memory, and movement in a C. elegans chemotaxis circuit. Neuron 2014;82(5):1115–28. pmid:24908490 - 24.
Jang MS, Toyoshima Y, Tomioka M, Kunitomo H, Iino Y. Multiple sensory neurons mediate starvation-dependent aversive navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2019;116(12):5807–12. - 25.
Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 1995;14(4):803–12. pmid:7718242 - 26.
Tanimoto Y, Yamazoe-Umemoto A, Fujita K, Kawazoe Y, Miyanishi Y, Yamazaki SJ, et al. Calcium dynamics regulating the timing of decision-making in C. elegans. Elife. 2017;6:e21629. pmid:28532547 - 27.
Thapliyal S, Beets I, Glauser DA. Multisite regulation integrates multimodal context in sensory circuits to control persistent behavioral states in C. elegans. Nat Commun 2023;14(1):3052. pmid:37236963 - 28.
Ji N, Venkatachalam V, Rodgers HD, Hung W, Kawano T, Clark CM, et al. Corollary discharge promotes a sustained motor state in a neural circuit for navigation. Elife. 2021;10:e68848. pmid:33880993 - 29.
Katzen A, Chung H-K, Harbaugh WT, Della Iacono C, Jackson N, Glater EE, et al. The nematode worm C. elegans chooses between bacterial foods as if maximizing economic utility. Elife. 2023;12:e69779. pmid:37096663 - 30.
Liu H, Wu T, Canales XG, Wu M, Choi M-K, Duan F, et al. Forgetting generates a novel state that is reactivatable. Sci Adv. 2022;8(6):eabi9071. pmid:35148188 - 31.
Chen KS, Wu R, Gershow MH, Leifer AM. Continuous odor profile monitoring to study olfactory navigation in small animals. Elife. 2023;12:e85910. pmid:37489570 - 32.
Tadres D, Wong PH, To T, Moehlis J, Louis M. Depolarization block in olfactory sensory neurons expands the dimensionality of odor encoding. Sci Adv. 2022;8(50):eade7209. pmid:36525486 - 33.
Gershow M, Berck M, Mathew D, Luo L, Kane EA, Carlson JR, et al. Controlling airborne cues to study small animal navigation. Nat Methods 2012;9(3):290–6. pmid:22245808 - 34.
Worthy SE, Haynes L, Chambers M, Bethune D, Kan E, Chung K, et al. Identification of attractive odorants released by preferred bacterial food found in the natural habitats of C. elegans. PLoS One 2018;13(7):e0201158. pmid:30036396 - 35.
Cho C, Brueggemann C, L’Etoile N, Bargmann C. Parallel encoding of sensory history and behavioral preference during Caenorhabditis elegans olfactory learning. eLife Sci. 2016;5:e14000. - 36.
Lin A, Qin S, Casademunt H, Wu M, Hung W, Cain G, et al. Functional imaging and quantification of multineuronal olfactory responses in C. elegans. Sci Adv. 2023;9(9):eade1249. pmid:36857454 - 37.
Gepner R, Mihovilovic Skanata M, Bernat NM, Kaplow M, Gershow M. Computations underlying Drosophila photo-taxis, odor-taxis, and multi-sensory integration. Elife. 2015;4:e06229. pmid:25945916 - 38.
Hernandez-Nunez L, Belina J, Klein M, Si G, Claus L, Carlson JR, et al. Reverse-correlation analysis of navigation dynamics in Drosophila larva using optogenetics. Elife. 2015;4:e06225. pmid:25942453 - 39.
Dahiya Y, Rose S, Thapliyal S, Bhardwaj S, Prasad M, Babu K. Differential regulation of innate and learned behavior by Creb1/Crh-1 in Caenorhabditis elegans. J Neurosci 2019;39(40):7934–46. pmid:31413073 - 40.
Gordus A, Pokala N, Levy S, Flavell SW, Bargmann CI. Feedback from network states generates variability in a probabilistic olfactory circuit. Cell 2015;161(2):215–27. pmid:25772698 - 41.
Kato S, Xu Y, Cho CE, Abbott LF, Bargmann CI. Temporal responses of C. elegans chemosensory neurons are preserved in behavioral dynamics. Neuron 2014;81(3):616–28. pmid:24440227 - 42.
Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 2007;450(7166):63–70. pmid:17972877 - 43.
Levy S, Bargmann CI. An adaptive-threshold mechanism for odor sensation and animal navigation. Neuron. 2020;105(3):534-548.e13. pmid:31761709 - 44.
Hendricks M, Ha H, Maffey N, Zhang Y. Compartmentalized calcium dynamics in a C. elegans interneuron encode head movement. Nature 2012;487(7405):99–103. pmid:22722842 - 45.
Chandra R, Farah F, Muñoz-Lobato F, Bokka A, Benedetti KL, Brueggemann C, et al. Sleep is required to consolidate odor memory and remodel olfactory synapses. Cell. 2023;186(13):2911-2928.e20. pmid:37269832 - 46.
Yoshida K, Hirotsu T, Tagawa T, Oda S, Wakabayashi T, Iino Y, et al. Odour concentration-dependent olfactory preference change in C. elegans. Nat Commun. 2012;3:739. pmid:22415830 - 47.
Omura DT, Clark DA, Samuel ADT, Horvitz HR. Dopamine signaling is essential for precise rates of locomotion by C. elegans. PLoS One 2012;7(6):e38649. pmid:22719914 - 48.
Dahlberg B, Izquierdo E. Contributions from parallel strategies for spatial orientation in C. elegans. ALIFE 2020: The 2020 Conference on. 2020. - 49.
Bartumeus F, Campos D, Ryu WS, Lloret-Cabot R, Méndez V, Catalan J. Foraging success under uncertainty: search tradeoffs and optimal space use. Ecol Lett 2016;19(11):1299–313. pmid:27634051 - 50.
Pillow JW, Shlens J, Paninski L, Sher A, Litke AM, Chichilnisky EJ, et al. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature 2008;454(7207):995–9. pmid:18650810 - 51.
Sharpee T, Rust NC, Bialek W. Analyzing neural responses to natural signals: maximally informative dimensions. Neural Comput 2004;16(2):223–50. pmid:15006095 - 52.
Lesar A, Tahir J, Wolk J, Gershow M. Switch-like and persistent memory formation in individual Drosophila larvae. Elife. 2021;10:e70317. pmid:34636720 - 53.
Tait C, Mattise-Lorenzen A, Lark A, Naug D. Interindividual variation in learning ability in honeybees. Behav Processes. 2019;167:103918. pmid:31351114 - 54.
Smith MA-Y, Honegger KS, Turner G, de Bivort B. Idiosyncratic learning performance in flies. Biol Lett 2022;18(2):20210424. pmid:35104427 - 55.
Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci 2010;13(5):615–21. pmid:20364145 - 56.
Ripoll-Sanchez L, Watteyne J, Sun H, Fernandez R, Taylor SR, Weinreb A, et al. The neuropeptidergic connectome of C. elegans. Neuron. 2023;111(22):3570–3589. - 57.
Calhoun AJ, Murthy M. Quantifying behavior to solve sensorimotor transformations: advances from worms and flies. Curr Opin Neurobiol. 2017;46:90–8. pmid:28850885 - 58.
Datta SR, Anderson DJ, Branson K, Perona P, Leifer A. Computational neuroethology: a call to action. Neuron 2019;104(1):11–24. pmid:31600508 - 59.
Nguyen JP, Shipley FB, Linder AN, Plummer GS, Liu M, Setru SU, et al. Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans. Proc Natl Acad Sci U S A 2016;113(8):E1074–81. pmid:26712014 - 60.
Roman A, Palanski K, Nemenman I, Ryu WS. A dynamical model of C. elegans thermal preference reveals independent excitatory and inhibitory learning pathways. Proc Natl Acad Sci U S A 2023;120(13):e2215191120. pmid:36940330 - 61.
Liu M, Sharma AK, Shaevitz JW, Leifer AM. Temporal processing and context dependency in Caenorhabditis elegans response to mechanosensation. Elife. 2018;7:e36419. pmid:29943731
ADVERTISEMENT:
sobat, pengemar slots Pernah mendengar istilah “slot gacor” Kalau? belum bersiaplah, hati jatuh dengan konsep slot gaco ini. merupakan slots mesin selalu yang kasih kemenangan Yup. mesin-mesin, disebut ini bisa adalah jagoannya buat bawa pulang cuan. tapi cemana, caranya sih jumpain slot demo benar yang Santai? Bro bahas, kita tenang saja di sini Games
tergacor waktu ini satu-satunya di hanya di Indonesia yang menyediakan ROI tertinggi SEGERA
dengan dengan di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia