Abstract
Bacteriophages infect gram-negative bacteria by attaching to molecules present on the bacterial surface, often lipopolysaccharides (LPS). Modification of LPS can lead to resistance to phage infection. In addition, LPS modifications can impact antibiotic susceptibility, allowing for phage–antibiotic synergism. The evolutionary mechanism(s) behind such synergistic interactions remain largely unclear. Here, we show that the presence of antibiotics can affect the evolution of resistance to phage infection, using phage ΦX174 and Escherichia coli C. We use a collection of 34 E. coli C LPS strains, each of which is resistant to ΦX174, and has either a “rough” or “deep rough” LPS phenotype. Growth of the bacterial strains with the deep rough phenotype is inhibited at low concentrations of chloramphenicol and, to a much lesser degree, gentamicin. Treating E. coli C wild type with ΦX174 and chloramphenicol eliminates the emergence of mutants with the deep rough phenotype, and thereby slows the evolution of resistance to phage infection. At slightly lower chloramphenicol concentrations, phage resistance rates are similar to those observed at high concentrations; yet, we show that the diversity of possible mutants is much larger than at higher chloramphenicol concentrations. These data suggest that specific antibiotic concentrations can lead to synergistic phage–antibiotic interactions that disappear at higher antibiotic concentrations. Overall, we show that the change in survival of various ΦX174-resistant E. coli C mutants in the presence of antibiotics can explain the observed phage–antibiotic synergism.
Citation: Parab L, Romeyer Dherbey J, Rivera N, Schwarz M, Gallie J, Bertels F (2025) Chloramphenicol and gentamicin reduce the evolution of resistance to phage ΦX174 by suppressing a subset of E. coli LPS mutants. PLoS Biol 23(1):
e3002952.
https://doi.org/10.1371/journal.pbio.3002952
Academic Editor: Jeremy J. Barr, Monash University, AUSTRALIA
Received: September 26, 2024; Accepted: November 25, 2024; Published: January 21, 2025
Copyright: © 2025 Parab et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Antibiotic susceptibility. Agar plate images and raw greyscale values (all replicates) used to generate Fig 2 and S1 Table are available in Edmond (https://doi.org/10.17617/3.PIDVUT). Whole genome re-sequencing. The E. coli C wildtype genome file (used as a reference), the ΦX174 wildtype genome (used as a reference), and raw sequencing reads of all sequenced phage-resistant E. coli C isolates are available in Edmond (https://doi.org/10.17617/3.PIDVUT). Bootstrap of fluctuation experiment. Raw bootstrap values used to plot error bars in Fig 5 and Fig S5 in S2 Text can be found in Edmond (https://doi.org/10.17617/3.PIDVUT) under file Figure5_bootstrap_observedmutations.xlsx. The code used for bootstrapping iterations and chi-squared test (Table 2) is available as chi_squared_test_models.R, and the input file for the code is available as chi_squared_test.xlsx in Edmond (https://doi.org/10.17617/3.PIDVUT). Two-day coevolution experiment. The 96-well plate-layout, growth curves for individual replicates and raw reads (growth data, source for Fig 6) for both days are available in Edmond (https://doi.org/10.17617/3.PIDVUT).
Funding: This work was generously supported by funds from the Max Planck Society (L.P.-F.B.). L.P. was supported by the International Max Planck Research School for Evolutionary Biology (IMPRS EvolBio). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations:
HSD,
Honest Significant Difference; LB,
lysogeny broth; LPS,
lipopolysaccharide; LRT,
likelihood-ratio test; MOI,
multiplicity of infection
Introduction
The rise of antibiotic-resistant bacteria is a global threat to public health [1]. To tackle this issue, several alternatives to antibiotics have been proposed [2]. Among the most promising of these are bacteriophages (phages), which have been successfully applied to a growing number of antibiotic-resistant bacterial infections [3–6]. However, as with antibiotics, a major reason for failure of phage treatments is the evolution of resistance [7]. The success of phages as therapeutic agents depends on our ability to limit the emergence of phage-resistant bacteria.
Reducing the evolution of phage-resistance in bacteria
One way to reduce the evolution of resistance is co-treatment of infections with specific combinations of phages and antibiotics [8–12]. Under these conditions, bacteria must evolve resistance to both the phage and the antibiotic to evade elimination. Interestingly, however, the efficacy of combined treatments is not always intuitive: examples of both synergistic and antagonistic interactions have been described. For example, phages NP1 and NP3 display synergistic effects with ceftazidime and ciprofloxacin when killing Pseudomonas aeruginosa biofilms [10]. Phages combined with antibiotics led to between a hundred-fold and thousand-fold higher killing rates, compared with phages alone. Contrastingly, phage SBW25Φ2 combined with streptomycin has been shown to increase rates of phage-resistance in Pseudomonas fluorescens populations [13]. While it is clear that the presence of antibiotics can affect the evolution of phage-resistance, thus far the underlying mechanisms remain largely unexplored. In this work, we provide a detailed example of how, and why, antibiotics can affect the course of phage-resistance evolution in bacterial populations.
Mechanisms of phage-resistance in bacteria
A common mechanism by which bacteria become resistant to phage is through the modification of cell surface components, such as lipopolysaccharide (LPS) molecules [14–16]. LPS molecules are found on the outer membrane of all gram-negative bacteria and are the most common phage receptor of gram-negative-infecting phages [17,18]. Most Escherichia coli strains produce a “smooth” LPS phenotype, consisting of a lipid A domain, a core oligosaccharide (consisting of an inner-core and an outer-core), and a polysaccharide called the O-antigen [19]. Some strains, such as E. coli C, lack the O-antigen and hence produce a “rough” LPS phenotype [20]. Further truncation of the LPS by removal of the outer-core oligosaccharide leads to a “deep rough” LPS phenotype [21].
E. coli C readily evolves resistance to ΦX174 through LPS alterations
Phage ΦX174 uses LPS to infect E. coli C [22]. ΦX174 is one of the oldest and most widely used phage model systems [23–25]. Surprisingly though, the mechanisms by which E. coli C can gain resistance to ΦX174—and how these can be overcome—have only been explored recently [16]. In this previous study, we showed that E. coli C populations readily become resistant to ΦX174 infection through mutations that generate truncated LPS structures (Fig 1) and that ΦX174 can evolve to overcome this type of resistance.
Fig 1. Predicted LPS phenotypes of 33 phage-resistant E. coli C strains.
Strains (R#) are categorised into “rough” (longer; outer-core present but modified, inner-core unmodified) or “deep-rough” (shorter; outer-core absent and/or inner-core modified), based on mutation location. LPS phenotype cannot be predicted for R25 as the mutated gene, rfaH, controls the expression of the entire waa LPS operon [31]. While the presence or absence of LPS outer core is commonly used to categorise LPS phenotypes, previous studies with gene deletion mutants show that absence of phosphate molecules in the inner-core also leads to a “deep rough” phenotype, as seen in R6 and R11 [20]. Kdo: 3-deoxy-D-manno-octulosonic acid; “?P”: No information was found on the phosphorylation of Hep(I) in the absence of waaF; (P): only 40% of the hexose phosphorylation was observed [32]. OS: oligosaccharide. Figure adapted from [16] with BioRender.com.
LPS phenotype affects sensitivity to both phage and antibiotics
In addition to affecting infection by phages, LPS phenotype can influence susceptibility to antibiotics. In E. coli, deep rough LPS mutants are more susceptible to membrane-targeting antibiotics such as colistin [26,27]. Resistance to many antibiotics that do not target the membrane can also be affected, because LPS modifications can also change membrane permeability [21,28]. For example, E. coli K-12 strains carrying a deep rough LPS structure are more susceptible to hydrophobic molecules such as novobiocin and spiramycin [21,28]. However, truncated LPS molecules can also lead to increased antibiotic-resistance. In P. aeruginosa, rough mutants (carrying mutations in galU) can increase antibiotic-resistance [29,30]. Hence, only some combinations of LPS-targeting phages, antibiotic, and target pathogen, are expected to reduce the evolution of resistance.
In the current study, we investigate the effects of antibiotics on the evolution of ΦX174-resistance in E. coli C. We begin by measuring the susceptibility of a set of 33 ΦX174-resistant E. coli C strains [16] to chloramphenicol and gentamicin. We find that E. coli C mutants with progressively shorter LPS molecules tend to be increasingly susceptible to both antibiotics (especially chloramphenicol). These results suggest that phage–antibiotic combination treatment prevents the growth of some antibiotic-resistant bacteria, and hence reduces the number and diversity of observable resistance mutations. The results of subsequent fluctuation experiments are consistent with this prediction; no E. coli C mutants with severely truncated LPS molecules were detected in a ΦX174+chloramphenicol combination environment. Finally, we use an E. coli C and ΦX174 co-evolution experiment in liquid media to show that, indeed, the ΦX174+chloramphenicol combination environment is the most efficient (of all combination treatments tested) at reducing phage-resistance evolution. Together, our results show that predicting phage-resistance rates in bacteria can be possible with surprising accuracy, using only very simple susceptibility assays.
Results
Phage-resistance mutations affect E. coli C susceptibility to chloramphenicol and gentamicin
The effect of LPS phenotype on antibiotic susceptibility is expected to depend on the antibiotic in question. Hence, in our first experiment, we investigated the effect of LPS phenotype on the susceptibility of E. coli C to two different antibiotics: chloramphenicol and gentamicin. These antibiotics inhibit protein synthesis via distinct mechanisms, respectively targeting the 50S and 30S ribosomal subunits [33]. We quantified the growth of 34 E. coli C strains with various predicted LPS phenotypes (see Fig 1) in the presence of increasing—but nonetheless low—concentrations of chloramphenicol or gentamicin on agar plates. The results are presented in Fig 2 and discussed in more detail below.
Fig 2. E. coli C deep rough LPS mutants show increased susceptibility to chloramphenicol and gentamicin.
Heatmaps show growth of E. coli C wild type and 33 E. coli C-derived, phage-resistant strains (R#) at increasing concentrations of chloramphenicol (left) and gentamicin (right). Growth is measured in 8-bit pixel greyscale values (see Methods) and decreases from violet (uniform lawn = greyscale values of 30 to 80) to yellow (no lawn = 0 to 8, and occasional colonies may be visible). White = not measured. Median greyscale values above 8 are defined as “growth” (left of bold black border), while values below 8 are defined as “no growth” (see Methods). Bacterial strains (rows) are ordered according to their predicted LPS phenotype (top to bottom: least to most truncated) and grouped as rough, undefined, or deep rough. Dotted horizontal lines group strains that have the same predicted LPS structure (shown on the left and indicated by letters a–h). *R27 and R31 have the same genotype (see Note A in S1 Text). The MIC of E. coli C wild type is approximately 4 μg/ml for both antibiotics. Increased bacterial growth at sub-MIC antibiotic concentrations is observed in some cases; similar observations have been described previously [35–38]. (B) Boxplots show the MIC of the phage-resistant E. coli C strains in chloramphenicol (left) and gentamicin (right). The average MIC of strains predicted to carry rough (longer) and deep rough (shorter) LPS molecules differs for both antibiotics (t test p = 4.15E-08 and 0.002, respectively; Wilcoxon rank sum test for tied data sets p = 1.05E-08 and 0.005, respectively). Bacterial strains predicted to display rough LPS are, on average, more susceptible to chloramphenicol and gentamicin. The data underlying this figure can be found in https://doi.org/10.17617/3.PIDVUT which includes agar plate images and greyscale values for each replicate (Batch_ROI_Grayscale_values.xlsx). Greyscale values are also plotted in Figs S1-S3 in S2 Text; median values used in panel A are available in S1 Table. Clipart for panel A created with BioRender.com.
Chloramphenicol.
The degree to which chloramphenicol affects E. coli C growth depends on LPS phenotype: progressively truncated LPS structures lead to increasing chloramphenicol susceptibility (Fig 2A and Fig S1 in S2 Text). More specifically, E. coli C strains displaying the rough LPS phenotype (including the wild type) showed no detectable growth at chloramphenicol concentrations of >3–4 μg/ml, while the growth of strains displaying the deep rough LPS phenotype was already inhibited at chloramphenicol concentrations of 2 μg/ml or less (Fig 2B and Fig S3 in S2 Text). Further, a subset of deep rough mutants predicted to possess the shortest LPS structures (R8, R10, R18, and R26) were most susceptible to chloramphenicol, with growth inhibition occurring at 1 μg/ml or lower.
Gentamicin.
Compared with chloramphenicol, susceptibility to gentamicin is less dependent on LPS phenotype. Many of the phage-resistant strains showed a similar growth profile to that of the wild type across the gentamicin concentrations tested, with significant growth inhibition first occurring at concentrations of 3 to 4 μg/ml (Fig 2A and 2B and Figs S2 and S3 in S2 Text). One exception was a subset of 6 strains with severely truncated LPS phenotypes (hldE/waaC mutants: R8, R10, R18, R26, R31, R32), the growth of which was inhibited at lower levels of gentamicin (1.5 to 2 μg/ml) (see also Note A in S1 Text). Interestingly, the growth of 5 strains—namely, 4 strain with the rough LPS phenotype (R34, R1, R33, R20) plus the strain of unknown LPS phenotype (R25)—was first inhibited at a slightly higher gentamicin concentration than the wild type (6 μg/ml versus 4 μg/ml). Hence, one might expect similar LPS mutations to be observed in gentamicin resistance screens. This is indeed the case for low-to-moderate levels of gentamicin [29,34].
The results in this section demonstrate that LPS phenotypes—and hence, phage-resistance genotypes—affect the susceptibility of E. coli C to both chloramphenicol and gentamicin. In general terms, shorter LPS structures tend to increase susceptibility to the antibiotic particularly chloramphenicol.
The presence of antibiotics is predicted to reduce diversity of phage-resistance mutations
In the previous section, we demonstrated that LPS mutations that decrease the susceptibility of E. coli C to ΦX174 can simultaneously increase susceptibility to low levels of antibiotics. This relationship suggests that the presence of antibiotics may affect the evolution of phage-resistance in E. coli C populations (and vice versa). Specifically, the frequency of phage-resistance mutations that render the bacterium more susceptible to antibiotic (i.e., those conferring the deep rough LPS phenotype) is expected to reduce in the presence of the antibiotic. In this section, we build a simple model to demonstrate this idea (model 1). This model can be outlined as follows:
- Phage-resistance is primarily caused by deactivating mutation(s) in gene(s) encoding the LPS biosynthetic machinery [16].
- If we assume that these deactivating mutations occur uniformly between, and across the length of, each LPS gene, the total length of the gene(s) giving rise to phage resistance (i.e., the mutational target size) can be inferred.
- Due to difference in which LPS phenotypes are permissible the mutational target size is affected by the presence—and concentration of—antibiotic. This information can be used to predict the relative rates of phage-resistance across environments.
Inferring permissible mutational target sizes.
The mutational target size can be inferred from the genes that contain loss-of-function mutations in the 33 phage-resistant strains [16]. Mutations in a total of 14 genes, with a combined length of 11,736 bp, led to phage-resistant mutants (Table 1). This mutational target size is specific to the ΦX174-only environment; as the antibiotic concentration increases, the number of genes in which phage-resistance mutations are accessible (i.e., lead to survival) decreases (see Fig 2). Such “inaccessible” genes can be deducted from the permissible target size in order to estimate the frequency of resistance to phage in each environment (Table 1).
The predicted relative resistance rates are provided in the final row of Table 1 (and graphically in Fig 3B, dark red points). Overall, as antibiotic levels increase, the relative rate of phage-resistance is predicted to decrease. Most notably, the phage-resistance rate in 2 μg/ml chloramphenicol is predicted to be less than half (47%) of that expected when treating the bacteria only with ΦX174. Combination treatment with gentamicin reduces the predicted resistance by only 20% at the highest concentration tested (2 μg/ml).
Fig 3. Antibiotics reduce the rate of resistance to ΦX174.
(A) Schematic of the fluctuation test and expected results. Adding chloramphenicol at subinhibitory concentrations is predicted to reduce rates of resistance to ΦX174, due to inhibition of the growth of deep rough LPS phenotypes. Gentamicin, however, is predicted to have a much lower effect on the rate of resistance to ΦX174. (B) Resistance rates (and 95% confidence intervals) for each phage+antibiotic plating environment, relative to the resistance rate in the ΦX174-only environment. Mean observed rates (black) are qualitatively in line with the predicted rates from model 1 (gene length model, dark red; derived in Table 1). Rates predicted by model 2 (gene proportion model inferred from the occurrence of mutations in a phage-only fluctuation test (see below), yellow; derived in S6 Table) are within 95% confidence intervals for gentamicin (GM), and 2% away from the 95% confidence interval in 2 μg/ml chloramphenicol (CL). Resistance rates are not predicted at antibiotic concentrations inhibitory for wild type (6 μg/ml CL, 4 μg/ml GM). The difference between models 1 and 2 can be mostly attributed to mutation bias (see text). The difference between model 2 and the observed resistance rate is most likely due to the effect size of phage–antibiotic interactions. (C) Absolute resistance rates (per cell division and 95% confidence intervals), including gentamicin at wild-type MIC without ΦX174. Rates of ΦX174 with antibiotic at inhibitory concentrations seem to be zero in panel B but are actually ~400–1,000-fold lower, as can be seen in panel C. In panel C, some 95% confidence intervals are too small to be readily visible. The data underlying this figure can be found in S2 Table (resistance rates and other experimental values used for calculating them; see Methods for details) and S3 Table (raw colony counts). Panel A created with BioRender.com.
Observed phage-resistance rates are qualitatively in line with a priori predictions
In order to test the relative phage-resistance rates predicted in the a priori model above, a classic fluctuation experiment was performed [39,40]. A total of 430 independent E. coli C wild-type populations were grown (without phage or antibiotics). Each grown population was spread onto LB agar plates containing either (set 1) ΦX174 alone, (set 2 to 4) ΦX174 in combination with chloramphenicol (at 1 μg/ml, 2 μg/ml, or 6 μg/ml), (set 5 to 7) ΦX174 in combination with gentamicin (at 1 μg/ml, 2 μg/ml, or 4 μg/ml), a total of 50 replicates for each of the 7 environments (Fig 3A). In addition, 6 μg/ml chloramphenicol (set 8, n = 30) and 4 μg/ml gentamicin (set 9, n = 50) were used as control environments. For each plating environment, the number of resistant bacterial colonies arising from each population was counted. The distributions of colony numbers were used to calculate the observed relative phage-resistance rates [41] (Fig 3B and 3C).
In model 1, the addition of chloramphenicol was predicted to reduce the rate of phage-resistance in E. coli C (Table 1). Indeed, the addition of chloramphenicol did significantly reduce relative phage-resistance rates (likelihood-ratio test (LRT) p −5 for both 1 μg/ml and 2 μg/ml) (S2 Table). However, the observed relative rates of phage-resistance were far lower than expected: 15% at 1 μg/ml (~4-fold lower than the predicted 86%) and 18% (~2-fold lower than the predicted 47%) (Fig 3B). The addition of gentamicin was predicted, and observed, to have no effect on phage resistance rates at 1 μg/ml gentamicin (LRT p = ~0.53). At 2 μg/ml gentamicin, the relative phage-resistance rate was reduced to 60%, somewhat further than was predicted (80%) (Table 1).
Overall, the addition of low concentrations of chloramphenicol or gentamicin did reduce the rate of phage-resistance in E. coli C (as predicted by model 1). However, the presence of antibiotics reduced the rate of phage-resistance much more effectively than predicted by the model. This could indicate that the reduction in rates of phage-resistance is not solely attributable to smaller mutational target sizes and that other factors should be considered for more accurate predictions. However, before considering additional factors, it is important to rule out the possibility that minor improvements to the existing model may lead to a better match with the observations.
Chloramphenicol skews mutations towards genes causing a rough LPS phenotype
So far, the results have demonstrated that combining ΦX174 with chloramphenicol and, to a lesser degree, gentamicin reduces the rate of phage-resistance in E. coli C. In model 1, we hypothesised that this is—at least partly—due to a reduction in the number of accessible mutations that lead to phage-resistance. To directly test this hypothesis, 222 phage-resistant E. coli C mutants were isolated from five of the fluctuation test plating environments, and each subjected to whole genome re-sequencing. A total of 239 mutations, belonging to a range of mutational classes, were identified (Fig 4 and Fig S4 in S2 Text and S4 Table). Of the 222 mutant strains, 194 were found to contain mutations in the 14 previously identified LPS genes [16], and two new LPS genes were identified as mutational targets (hldD and gmhA). Some degree of parallel evolution (identical mutations) was observed, both within and between plating environments (Note B in S1 Text). We note that, in four mutant strains, no putative mutations were identified (possibly due to low coverage of the LPS biosynthetic operon, see S4 Table).
Fig 4. The presence of antibiotics reduces mutational target size for phage-resistance evolution in E. coli C.
(A) Genomic distribution of phage-resistance mutations in E. coli C, under 5 plating environments of the fluctuation test (rows). Large deletions (>100 bp) are indicated by solid horizontal lines scaled to the deleted region; all other classes of mutations are indicated as dashes. Red boxes indicate loci in which mutations are absent (or extremely rare) under some environments. Genes contributing to LPS inner core synthesis are underlined and in red; loss-of-function mutations in these genes are predicted to give a deep rough LPS phenotype. *RfaH is responsible for regulation of the LPS operon, and rfaH mutations can lead to a mixture of rough and deep rough types [31,42]. (B) Gene-wise distribution of the phage-resistance mutations observed in the absence of, and presence of 2 μg/ml, antibiotics. Bars show the proportion of phage-resistant E. coli C mutants with a mutation in the specified gene (ΦX174-only n = 49, ΦX174+CL(1 μg/ml) n = 39, ΦX174+CL(2 μg/ml) n = 36, ΦX174+GM(1 μg/ml) n = 46, ΦX174+GM(2 μg/ml) n = 42). CL: chloramphenicol, GM: gentamicin. The raw data underlying this figure can be found in S4 Table.
Combining phage and antibiotics biases mutations towards rough LPS genes (Fig 5A).
Mutations in strains resistant to phage treatment alone are predominantly found in deep rough LPS genes (approximately 86%), and only about approximately 12% are in rough LPS genes. As expected from our predictions, adding chloramphenicol enriches for rough mutants. At 1 μg/ml chloramphenicol, the proportion of rough mutants increases from ~12% to ~62%, and the proportion of deep rough mutants decreases from ~86% to ~36%. At a concentration of 2 μg/ml chloramphenicol, all mutants are rough mutants. This result was somewhat surprising, since we predicted to observe at least some deep rough mutants (~11%) (Fig 5A). Gentamicin treatment also leads to a smaller proportion of deep rough mutants, but as expected from our predictions, the effect is much smaller. At a concentration of 1 μg/ml gentamicin deep rough mutants remain largely stable at ~85% and ~15% of the mutants are rough. At 2 μg/ml gentamicin, the ratio becomes more skewed towards a rough LPS with about 50% deep rough mutants and ~43% rough mutants (Fig 5A).
Fig 5. Chloramphenicol heavily skews phage-resistance mutations towards rough LPS loci.
(A) Bars show the percentage of phage-resistant E. coli C mutants that carry mutations predicted to give rise to the rough or deep rough LPS phenotype, under various fluctuation test plating environments. Error bars show the standard deviation for each LPS type from 100 bootstrapping replicates (resampling with replacement, see Methods). Model 1 predictions (dark red points) assume that the probability of mutation is proportional to gene length (Table 1). In model 2 (dark yellow points), the probability of mutation is based on the observed proportions in the ΦX174-only environment (S6 Table). Predictions for ΦX174+antibiotic environments are based on the susceptibility data (see Fig 2). Mutations in rfaH can affect any number of inner or outer core genes, and hence are in a separate LPS phenotype category. (B) Gene-wise distribution of phage–resistance mutations, in the absence of any antibiotics. The proportions predicted by model 2 differ from the observed proportions in the ΦX174-only environment because we add a pseudo-count of 1 to counts for each of the genes, to allow statistical handling of zero values (see Methods). “Non LPS” refers to genes whose potential involvement in LPS biosynthesis, assembly, or regulation remains uncharacterized (4 genes across all experiments, see Note C in S1 Text). Error bars show the standard deviation from 100 bootstrap replicates for each gene (B, C). (C) Gene-wise distribution of phage–resistance mutations, in the ΦX174+chloramphenicol (2 μg/ml) environment. As outlined in Model 2, rfaH, galU, and waaP mutants are predicted to survive, but none were observed. (See Fig S5 in S2 Text for model 3.) The data underlying this figure can be found in S4–S7 Tables, which include raw mutation data as well as summarised values. Raw bootstrapping values can be found in the file Figure5_bootstrap_observedmutations.xlsx in https://doi.org/10.17617/3.PIDVUT.
Adjusted model improves predictions and highlights potential phage–antibiotic interactions
Before attempting to predict outcomes of combination environments, our model should be able to accurately predict outcomes in the ΦX174-only environment. In the ΦX174-only environment, deep rough mutants were observed at significantly higher rates (86%) than predicted from the mutational target size (model 1, 58%) (Fig 5A and S5 Table). One possible explanation for this mismatch is that the distribution of mutations is not actually proportional to gene length, as model 1 assumes. For example, mutational bias could lead to unexpectedly high (or low) rates of particular mutations [43,44]. To investigate this possibility, we used the proportion of mutants observed in each locus, in the ΦX174-only environment of the fluctuation test (a pseudo-count of 1 was added to each gene, to avoid false negatives, see Methods) (S6 Table). The adjusted predicted proportions are shown in yellow in Fig 5B (model 2). Adjusting the baseline predictions should, in theory, improve the predictions for the combination environments.
Using model 2 quantitatively improves predicted resistance rates (see Fig 3B), especially for environments with 2 μg/ml antibiotic (and phage). The new predicted resistance rate for 2 μg/ml gentamicin (61%) is now within the 95% confidence interval and close to the measured resistance rate (60%, with 95% CI 50% to 70%), while the predicted resistance rate in the 2 μg/ml chloramphenicol environment (i.e., 25%) now lies almost within the 95% confidence interval (18%, with 95% CI of 14% to 23%). However, the model 2-predicted distribution of rough and deep rough mutants fits the observed distribution less well than the model 1 predictions do (Fig 5A). The only environment where model 2 improved predictions of LPS mutant distribution above those in model 1 is ΦX174+1 μg/ml gentamicin (the environment closest to the ΦX174-only environment, based on our initial susceptibility assay) (Fig 5A). Model 2 also allows us to more accurately predict the effect of phage–antibiotic interactions on resistance rates by more accurately accounting for mutation bias (as shown in Fig 3B). The only environment where phage–antibiotic interactions appear to play a significant role is in the ΦX174+1 μg/ml chloramphenicol environment.
Model 2 qualitatively predicts the shift towards rough mutants, but without quantitative accuracy (Fig 5A). Since some waaP and galU mutants grow at 2 μg/ml chloramphenicol in our susceptibility assays, deep rough waaP and galU mutants are predicted to occur at a rate of 6% and 11%, leading to a non-zero deep rough prediction (Fig 5A and 5C and S6 Table). RfaH mutants are predicted to occur at a rate of 13%, because they also grow well in our susceptibility assay. Yet, waaP, galU, and rfaH mutants were not observed at 2 μg/ml chloramphenicol (Fig 5C). Similarly, at 1 μg/ml chloramphenicol, mutations are predicted in rfaH, waaF, gmhB, and gmhA but none were observed (panel C of Fig S5 in S2 Text). At 2 μg/ml gentamicin, mutations are predicted in hldE, gmhA, and gmhB (deactivation of either is predicted to result in the deep rough LPS phenotype), but none were observed (panel D of Fig S5 in S2 Text).
There are at least three possible reasons for the above discrepancies between predictions and observations. First, sampling only 40 to 50 mutants per environment could under-sample the proportion of mutated LPS genes. Sampling more mutants in the ΦX174-only environment may improve the accuracy of predicted proportions (and prevent the need for the addition of pseudo-counts in our models). Second, biological interactions between antibiotics and phages [45,46] may lead to the overrepresentation of different LPS genes compared to the ΦX174-only environment. Third, it is possible that what is considered growth in our susceptibility assay is not sufficient to be detected in the fluctuation experiment.
We are planning to test hypotheses 1 and 2 in future studies, hypothesis 3 can be tested by adjusting the threshold for growth in our susceptibility assay. When we increase the threshold for growth from a greyscale value of 8 to 12 (model 3), phage-resistance rate predictions remain largely unchanged from those in model 2, with the exception of the 2 μg/ml gentamicin environment, where the prediction worsens (panel A of Fig S5 in S2 Text). Predicted distributions across LPS genes do not change significantly for most environments (panel B of Fig S5 in S2 Text and S6–S7 Tables), but model 3 does indeed improve the gene-wise predictions for phage+2 μg/ml antibiotic (panels D and E of Fig S5 in S2 Text). With the higher threshold, gmhB, gmhA, and hldE mutants are no longer predicted to grow at 2 μg/ml gentamicin (panel D of Fig S5 in S2 Text), neither are waaP and galU mutants at 2 μg/ml of chloramphenicol (panel E of Fig S5 in S2 Text), which matches our experimental observations. However, none of the model adjustments explain the discrepancy between the predicted and observed phage-resistance rates, or proportions of deep rough and rough mutants at 1 μg/ml chloramphenicol (panels A and B of Fig S5 in S2 Text). Hence, this discrepancy is most likely the result of direct or indirect interactions between phages and chloramphenicol.
Interactions between phages and chloramphenicol are also indicated by statistical comparisons between observations and expectations according to different models (Table 2). If there is no statistical difference between model and observation, we assume that the model performs well. As the difference between model and observation becomes more significant, the model fits the observations less well. Of the three models, models 2 and 3 provide the most accurate predictions of rough and deep rough mutant proportions (Table 2), and phage-resistance rates (Fig 3B and Fig S5 in S2 Text). The opposite is true for gene-wise distribution predictions, since model 1 is closer to the observations than later models (with the exception of the ΦX174+1 μg/ml gentamicin environment). This indicates that gene length is a reasonable proxy for the likelihood of observing a mutation in a particular gene (bottom of Table 2). Hence, adjusting the mutation proportions to the ΦX174-only environment leads to fewer errors for resistance rate and LPS type predictions, but greater errors for gene-wise predictions.
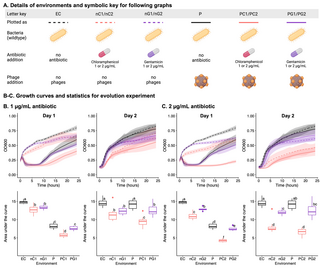
Chloramphenicol addition leads to strongest synergism in co-evolving E. coli C-ΦX174 populations
To test whether our above predictions also hold in more complex environments, we set up a serial transfer co-evolution experiment. The experiment began with ten distinct E. coli C-ΦX174-antibiotic combinations (see Fig 6A). The growth of 12 replicate cultures in each environment was recorded for 24 h (day 1), after which 2% of each culture was transferred to fresh media (with the same antibiotic concentrations as day 1) and growth continued for a further 24 h (day 2). The most influential factor in the growth of day 1 cultures was the presence of phages; as expected, cultures with phages grew significantly more slowly than those without phages (Fig 6B and 6C, left panels). However, even cultures containing phages showed considerable bacterial growth by 24 h, suggesting the rapid emergence of phage-resistant bacteria (including the emergence of phage-resistant genotypes). Indeed, on day 2, the presence of phages is no longer the most influential factor on growth of the bacterial population (Fig 6B and 6C, right panels). Instead, the growth profiles of day 2 populations group not by phage presence, but instead by antibiotic: cultures with no antibiotic grew most quickly, then those with gentamicin, and finally those with chloramphenicol. Antibiotics becoming the dominating factor in our evolution experiment at day 2 is strong evidence of rapid phage resistance evolution. Further, the course of evolution is affected by the presence of antibiotics: phage resistance emerges rapidly on day 1 when no antibiotic is present, but considerably more slowly when chloramphenicol is present. In 2 μg/ml gentamicin, phage resistance emerges without a delay, yet bacterial growth is significantly inhibited. On day 2 for high antibiotic concentrations, there is no significant difference between the antibiotic only and the antibiotic-phage treatments except for the 1 μg/ml chloramphenicol environment.
Fig 6. Chloramphenicol affects co-evolution of E. coli C and ΦX174.
(A) Details of the 10 distinct E. coli C culture environments in the 2-day evolution experiment, containing the indicated combinations of ΦX174, chloramphenicol, and/or gentamicin (n = 12 per environment). Day 2 populations were founded by transferring 2% of each population into fresh media under the same conditions as day 1. (B, C) The effect of 1 μg/ml (B) or 2 μg/ml (C) antibiotic on the growth of E. coli C cultures with and without ΦX174. Growth is presented as absorbance measures over time (top) and area-under-the-curve (AUC, bottom), on day 1 (left), and day 2 (right). Letters on the AUC plots denote statistical significance groups for each day (a–f): there is no statistically significant difference between environments that share a particular letter on one day, while there is a significant difference between environments that do not share the letter. For example, on day 1, in 1 μg/ml antibiotic (B), there is no significant difference between nC1 and nG1, or between P and PG1, but all other pair-wise combinations are significantly different (Tukey’s Honest Significant Difference test, see Methods). Statistical testing was done separately for (B) and (C). The data underlying this figure can be found in https://doi.org/10.17617/3.PIDVUT in the file Figure6_Platereader_raw_data.xlsx. Panel A created with BioRender.com.
Only for the 1 μg/ml chloramphenicol environment remain phages a significant growth inhibitor on day 2 (Fig 6B). This result is in line with the low level of phage resistance evolution at 1 μg/ml chloramphenicol in the solid fluctuation experiment (Fig 3B). Similarly, we posit that interactions between phages and antibiotics are likely the cause for the continued efficacy of phage treatment and prevent the emergence of fast-growing phage-resistant mutants.
Discussion
Here, we find that phage–antibiotic combinations can reduce the evolution of phage-resistance in bacteria, by inhibiting the growth of certain bacterial mutants. We start by quantifying the effect of chloramphenicol and gentamicin on the growth of 34 previously isolated ΦX174-resistant E. coli C strains. From these measurements, we predict that certain classes of mutations will not emerge in specified phage–antibiotic combination treatments, and hence these combination treatments will reduce the rate of phage-resistance evolution. We test these predictions by performing an experiment where we expose E. coli C wild type to phage ΦX174 and antibiotics. The observed phage resistance rates generally agree with our predictions and whole-genome sequencing of phage-resistant mutants support our predictions of mutational landscape accessibility. Overall, we show that antibiotics can reduce phage resistance evolution by preventing the growth of deep rough LPS resistance types. This effect is often referred to as an evolutionary trap, and can be useful for evolutionary steering [27,47,48]. Interestingly, first order effects—effects which do not take interactions between phage and antibiotic into account—are largely sufficient to explain reduced phage resistance evolution in combination treatments. Finally, we show that our predictions hold even in a more complex co-evolution experiment, where phage-chloramphenicol combinations are the most potent treatments for slowing bacterial growth.
Phage–chloramphenicol interactions likely amplify the effect of combination treatments
The original model assumption for the fluctuation assay posits that the outcome of the assay only depends on 2 parameters: the mutation rate and the mutational target size [39]. The mutation rate should be identical across all treatments, since mutations occur during exponential growth in liquid culture in the absence of antibiotic and phages. The second parameter, the measured mutational target size, is very close to our expectations for 1 μg/ml chloramphenicol and similar to the measured target size for 2 μg/ml gentamicin (Fig 4A). Yet, for gentamicin the predicted phage resistance rate (61%) fits well with the observed resistance rate (60%), while for chloramphenicol the rates differ significantly (64% predicted versus 15% observed, Fig 3B). Given that target size and mutation rates are identical between the environments, there must be other factors that explain our observations.
One possible explanation is that mutations do not guarantee resistance. It is possible that for 1 μg/ml chloramphenicol the mutations only provide a certain survival chance rather than guaranteed survival. Previous studies have shown that while low concentrations of chloramphenicol do not lead to stochastic population extinctions, combination of chloramphenicol and a bactericidal antibiotic does [49]. Given that phages can be considered bactericidal agents, stochastic population extinctions could play a role in our experiment. If the stochastic extinction chance is around 50%, then the expected resistance rate would correspond to our observed resistance rate. This stochastic survival effect may result from several possible factors, including physiological interactions between phage and chloramphenicol on the plate, local fluctuations in chloramphenicol concentration, or differences in physiological cell state [50].
The combination between a bactericidal agent (phage) and a bacteriostatic antibiotic (chloramphenicol) may lead to a trade-off explaining why phage resistance rates at 1 μg/ml and 2 μg/ml chloramphenicol are similar. At high chloramphenicol concentrations, the antibiotic can efficiently prevent the emergence of deep rough mutants. However, the efficiency of killing by the phage may be simultaneously compromised because of reduced bacterial growth. Slower growing bacteria inhibit phage infection and hence phage killing efficacy [49,51]. At slightly lower concentrations, chloramphenicol has a smaller effect on bacterial growth, rendering phage infection more efficient. These effects could conceivably balance each other out, leading to similar phage resistance rates at chloramphenicol concentrations of 1 μg/ml and 2 μg/ml.
Similar to our results in solid media, we also observe the strongest effect of combining phage and antibiotic in the 1 μg/ml chloramphenicol environment. This phage–antibiotic combination is the only environment where phages remain potent even on day 2 of the experiment. We propose that the interaction between antibiotic and phage prevent the evolution of phage resistance. The mechanism behind this interaction remains to be uncovered.
Mutants resistant to phage-only treatment are biased towards deep rough LPS
In the phage-only environment, mutants with a deep rough LPS emerge at a higher proportion than expected based on the assumption that mutations occur in proportion to gene length. We observed that 86% of all mutants display the deep rough LPS phenotype (compared to the expected 58%, based on gene length) (Fig 5B). It is unclear why deep rough mutants are overrepresented, but one reason could be that mutation rates are not uniform throughout the genome. It is possible that there are many loci with high mutation rates in genes that cause deep rough phenotypes (mutational bias) [43,52,53]. Similarly, deep rough LPS candidate genes may contain a higher number of mutational targets (i.e., loci that produce a phage-resistant LPS phenotype) than do rough LPS candidate genes. Alternatively, mutations in deep rough LPS genes may be more likely to produce robust resistance—the ability of the mutation to reliably result in the establishment of a colony—in the presence of phages alone.
While deep rough LPS mutants are more abundant—at least in E. coli—they are more susceptible to certain antibiotics [21,54]. This characteristic could potentially be exploited by complementing phage therapy with antibiotics that specifically affect bacterial strains with a deep rough LPS structure.
LPS modifications affect susceptibility to chloramphenicol and, to a lesser degree, gentamicin
Chloramphenicol and gentamicin are similar in their mechanism of action [33] and resistance mechanism [29,55–57]. Thus, the reason that chloramphenicol affects deep rough mutants differently may hinge on its physicochemical properties. The two antibiotics differ in their type of inhibition, hydrophobicity, and molecular size. Specifically, chloramphenicol is a bacteriostatic, hydrophobic molecule of 323 Da, while gentamicin is a bactericidal, hydrophilic molecule of 477 Da. The key differences are most likely hydrophobicity and size, because small hydrophobic molecules can diffuse through the LPS barrier, while hydrophilic molecules generally require active transport for cell entry [28].
The increased susceptibility of deep rough LPS mutants to hydrophobic antibiotics is in line with previous studies. Deleting deep rough LPS genes (waaC, waaF, waaG, or waaP) increases membrane hydrophobicity and permeability [54] can destabilise the membrane and increases the entry of small hydrophobic molecules [21,58,59]. Testing more antibiotics could reveal whether type of inhibition, molecular size, or hydrophobicity has the strongest effect on susceptibility.
Host range extension experiments could make ΦX174 a potent therapeutic agent
Understanding the combined effect of phages and antibiotics on bacterial evolution ultimately aims to improve therapeutic outcomes. Unfortunately, ΦX174 itself is currently not a therapeutically relevant phage since it infects only about 0.8% of 783 E. coli human and animal commensal strains [22], and no pathogenic strains. However, the host range of ΦX174 can be expanded through evolution experiments [16,60] or genetic engineering [61]. Once the limitation of phage entry is overcome, ΦX174 can replicate its DNA and successfully lyse hosts, including various E. coli strains and other species such as S. enterica and Klebsiella aerogenes [62–64]. Extending the host range of ΦX174 could allow the extensive knowledge of ΦX174 biology accumulated over the last few decades to be used to convert ΦX174 into an efficient therapeutic agent, especially when combined with antibiotics to exploit shortened LPS structures [60].
More generally, our results show that phages used in combination with low levels of certain antibiotics can reduce the evolution of phage resistance in bacteria. This result indicates that the continued use of antibiotics may be beneficial even when the target bacteria are antibiotic-resistant. Of course, further studies are required to test whether our results also hold for higher, more clinically relevant antibiotic concentrations and resistance rates.
LPS modifications may also reduce pathogen virulence when treating with LPS-targeting phages. LPS can increase virulence [65–67] and elicit immune responses [68]. If the evolution of phage-resistance leads to the truncation of LPS structures, then developing resistance to phage infection may render the pathogen less virulent. Future experiments with clinically relevant antibiotics and phages should be conducted to allow us to understand which LPS-targeting phage–antibiotic combinations are most beneficial in therapeutic settings.
Methods
Bacteria and phage used in this study
E. coli C wild type and phage ΦX174 (GenBank accession number AF176034) were provided by H.A. Wichman (University of Idaho, United States of America). The E. coli C wild type used in this study differs from the NCBI E. coli C strain (GenBank accession number CP020543.1) by 9 insertions and 2 nucleotide replacements (see [16]). E. coli C phage-resistant strains R1 through R35 were generated in a previous study [16]; all other phage-resistant strains were generated in this study.
Media and growth conditions
Bacteria and phages were grown in lysogeny broth (LB, Miller) supplemented with 5 mM CaCl2 and 10 mM MgCl2 to (henceforth referred to as “supplemented LB”). Supplemented LB agar (1.5%) was used to plate phages and bacteria. Plates were incubated for approximately 17 h at 37°C. Where appropriate, chloramphenicol or gentamicin was added at the concentration indicated. For bacterial overnight cultures, 5 ml supplemented LB in a 13 ml culture tube (Sarstedt) was inoculated with a single colony from a supplemented LB agar plate and incubated for approximately 17 h (37°C, 250 rpm). Fresh ΦX174 stocks were prepared weekly, using E. coli C wild-type cultures in exponential phase as described previously [16].
Determination of antibiotic susceptibility of bacterial strains
Determination of MIC.
Preliminary measurements of the antibiotic susceptibility of E. coli C R1 through R35 were obtained using ETEST strips (bioMérieux, France) on supplemented LB agar plates, in order to narrow down the range of concentrations to be tested for MIC determination. Overnight cultures of E. coli C wild type and phage-resistant strains were diluted 10-fold in Ringer’s solution; 50 μl of each diluted culture (approximately 5 × 106 cells) was spread, using approximately 15 glass beads (4 mm diameter), on a 90 mm supplemented LB agar plate (with or without antibiotic, as indicated). Plates were incubated at 37°C overnight (approximately 17 h). The next day, plates were cooled to room temperature and imaged for growth assessment. If bacterial growth was present on the images of all tested concentrations, a new round of testing with increased antibiotic concentrations was instigated. Tests were continued until there was no detectable growth across 3 replicates [69].
Imaging to measure growth/susceptibility.
Supplemented LB agar plates were uniformly photographed using a Chemidoc imaging system (Bio-Rad) under the colorimetric setting with an exposure of 1 s (see Data Availability). Images were analysed using OMERO (web server, version 5.14.1) to quantify growth [70]. For uniform measurements, a rectangular region with a constant area (1,157.85 mm2, an area size free of light glare in most images) was marked on each image. Growth was measured in 8-bit pixel greyscale values from 0 to 255. Growth measures were obtained by subtracting the average greyscale value of a blank plate from the average greyscale value of each sample plate (S1 Table). A uniform bacterial lawn corresponded to greyscale values of 30 through 80 (depending on the thickness and density of the lawn). No, or very little, visible growth corresponded with greyscale values of 0 through 8. Average greyscale values of more than 8 are defined as “growth,” and values of less than 8 are defined as “no growth.” The sensitivity of the assay to the growth cut-off was tested by changing the growth cut-off to 12 in model 3 (S7 Table). Median growth values across all (1 to 5) biological replicates were used for Fig 2. The lowest concentration at which the median value was less than the threshold (8 or 12) was considered the MIC.
Fluctuation test and isolation of phage-resistant E. coli C mutants
Phage-resistance rates were measured using a revised Luria–Delbrück fluctuation test [39,40], across 9 plating environments (n = 50 per environment): (set 1) ΦX174 alone; (sets 2 to 4) ΦX174 in combination with chloramphenicol (at 1 μg/ml, 2 μg/ml, or 6 μg/ml); (sets 5 to 7) ΦX174 in combination with gentamicin (at 2 μg/ml, 2 μg/ml, or 4 μg/ml); (set 8) 6 μg/ml chloramphenicol alone (exception, n = 30); (set 9) 4 μg/ml gentamicin.
For each environment, 50 single E. coli C wild-type colonies picked from supplemented LB agar plates. Each colony was inoculated, in its entirety, into 100 μl of supplemented LB without antibiotic or phage (in 96-well plates). Inoculated cultures were incubated for 5 h (37°C, 750 rpm). Cultures were resuspended by pipetting at the 3-h and 4-h time points to avoid settling from bacterial aggregation. After incubation, 50 μl of culture from each well was spread onto the corresponding supplemented LB agar plate. For all environments involving ΦX174, bacterial cultures were mixed with 50 μl of a high-titre phage lysate (~3 × 108 PFU/ml) prior to plating. Following initial incubation, plates with chloramphenicol at 6 μg/ml (with and without phage) were incubated further for 16 h to check for slow-growing colonies.
We designed the experiment to be able to accurately measure rates from 10−6 per cell division to 10−10 per cell division. Specifically, the amount of bacterial culture to be plated on selection plates was altered to have a countable number of resistant colonies [40,41]. Using a pilot experiment, we determined the size of bacterial culture to be plated for the expected number of colonies to be above 1 but below 100. For environment (set 1), 50 μl of 2-fold diluted bacterial culture was mixed with 50 μl of ΦX174 lysate; (sets 2 to 4) and (sets 5 to 7), 50 μl of undiluted bacterial culture (~3 × 108 CFU/ml) was mixed with 50 μl of ΦX174 lysate; for (set 8) and (set 9), 50 μl of 10-fold diluted bacterial culture was taken. The entire mixture was then plated on agar plates with the corresponding selective condition. For obtaining total CFU counts, cultures were serially diluted and plated on non-selective supplemented LB agar plates.
The next day, colonies were counted and 1 colony was randomly chosen from each replicate for which colonies were observed. Colonies were purified on non-selective supplemented LB agar plates, grown up in liquid culture, and stored at −80°C. In total, 222 mutants were stored in this way (ΦX174-only n = 50; ΦX174+CL(1 μg/ml) n = 41; ΦX174+CL(2 μg/ml) n = 37; ΦX174+GM(1 μg/ml) n = 46; ΦX174+GM(2 μg/ml) n = 43; ΦX174+GM(4 μg/ml) n = 1; ΦX174+CL(6 μg/ml) n = 4).
Resistance rate calculations and statistical analyses
Phage-resistance frequencies in E. coli C (i.e., the number of resistant colonies across 50 independent replicates) were used to calculate resistance rates. Resistance rates were calculated by the Lea–Coulson model following the method of Foster [71]. This method was used because (i) the bacterial populations were each founded by a single cell; (ii) the model assumes that the growth rates of mutants and non-mutants are the same in the absence of selection; and (iii) the model allows for partial plating of cultures. The resistance rate and 95% confidence interval were estimated using the Lea–Coulson (εwebSalvador (the web-application of R package rSalvador) [41]. Resistance rates were compared between different plating environments using the Lea–Coulson (εrSalvador (RStudio version 2022.02.1) [72,73].
Whole genome re-sequencing
Samples were prepared for whole genome re-sequencing from 500 μl of overnight culture, using a DNeasy Blood and Tissue kit (Qiagen). Samples were sequenced at the Max Planck Institute for Evolutionary Biology (Plön, Germany) with a NextSeq 500, using a NextSeq 500/550 High Output Kit v2.5 (300 Cycles) kit (Illumina). Output quality was controlled using FastQC version 0.11.9 [74], read-trimmed using Trimmomatic [75], and analysed using breseq [76]. Aside from SNPs, other predicted mutations were confirmed by mapping raw reads to the corresponding modified genome in Geneious Prime (2023.1.1, https://www.geneious.com).
Predicting phage-resistance rates
Model 1.
The probability of a ΦX174-resistance mutation occurring in a specific E. coli C gene is assumed to be proportional to the length of the gene’s coding region (Table 1). We assumed that only genes previously observed [16] confer resistance to ΦX174. In cases where a phage-resistant E. coli C mutant carried multiple mutations, only the mutation predicted to affect LPS structure was considered (e.g., waaO for R29, galE for R1).
The observed ability of phage-resistant mutants to grow in the presence of chloramphenicol or gentamicin was used to predict (i) phage-resistance rates; and (ii) genes in which mutations can confer resistance in phage–antibiotic combination environments (Fig 2 and values in S1 Table). If, out of “n” mutants with a mutation in a gene, only “x” mutants grew in the presence of the antibiotic, (x/n) was assumed to be the probability that this gene confers resistance in a combination environment. Hence, phage-resistance caused by a mutation in such a gene is proportional to (x/n)* gene length (i.e., a weighted score). For example, out of 5 galU mutants, only one can grow at 2 μg/ml chloramphenicol. Thus, resistance caused by a mutation in galU (909 bp) has a weighted score of 182 (1/5*909) in ΦX174+chloramphenicol (2 μg/ml).
Resistance rate is proportional to the sum of all weighted scores of genes conferring resistance. The relative resistance rate for a combination environment is inferred as a ratio of the total weighted scores under each selective condition to that of the ΦX174-only environment (Table 1).
Model 2.
The weighted scores assigned to each gene were changed to the number of mutations actually observed in the ΦX174-only environment (S6 Table). Mutations in 2 genes (gmhB and galE) are not observed in the ΦX174-only environment, meaning a 0 frequency, and 0 weighted score, in model 2. A weight of 0 incorrectly classifies these genes as not conferring phage-resistance and will also predict 0 probabilities for combination environments. This issue was solved by adding a pseudo-count of 1 to all genes, including galE and gmhB (green highlight in S6 Table).
The inverse problem exists for hldD and gmhA. These genes were not observed in [16]; hence the degree of antibiotic susceptibility incurred through mutations in these genes was not measured in Fig 2. Hence, phage-resistance rates and LPS type/gene distributions were calculated for both phenotypes. For final analyses, the model closest to the experimental observations (Fig 2) was used. GmhA mutants were assumed to grow similarly to gmhB mutant (i.e., growth in all environments except ΦX174+chloramphenicol (2 μg/ml)).
Model 3.
The growth cut-off greyscale value was increased from 8-bit to 12-bit (see Methods: section “Determination of antibiotic susceptibility of bacterial mutants”). Candidate genes and weighted scores of the baseline were the same as in model 2 (S7 Table).
Note: When comparing the 3 models with each other, model 1 was modified to include hldD and gmhA to avoid the statistical problem of zero expectation for any category (see “model 1 (added genes)” in Table 2).
Bootstrap analysis
A bootstrap analysis was performed to estimate sampling error in the 217 E. coli C strains isolated from phage–antibiotic combination environments (only 1 or 2 μg/ml antibiotic, hence 5 mutants from other environments were excluded). Mutants were re-sampled with replacement 100 times for each environment, in order to calculate the expected variation in the sampled observation. To re-sample genes in which mutations were not observed, but are nonetheless expected to confer phage-resistance when mutated, a pseudo-count of 1 was added to each gene that could provide phage-resistance in that environment (before sampling). The standard deviation of the 100 sampling events was used to draw error bars for both gene-wise and LPS-type distributions (Fig 5 and Fig S5 in S2 Text).
Co-evolution experiment
Environments.
In a 2-day co-culture experiment, we measured how cultures of E. coli C (and its resistant mutants) grow when exposed to phage–antibiotic combinations, compared to the presence of either phages or antibiotics alone. Growth was measured across 10 environments: (sets 1 and 2) ΦX174 with chloramphenicol at 1 μg/ml or 2 μg/ml; (sets 3 and 4) ΦX174 with gentamicin at 1 μg/ml or 2 μg/ml; (set 5) ΦX174 only; (sets 6 and 7) chloramphenicol only, at 1 μg/ml or 2 μg/ml; (sets 8 and 9) gentamicin only, at 1 μg/ml or 2 μg/ml; (sets 10) no phage or antibiotic. We repeated the whole experiment twice and combined the data in the final plots, resulting in a total of 12 replicates for each environment (Fig 6).
Day 1.
Six independent overnight cultures of E. coli C wild type were used to inoculate 6 pre-cultures, by adding 100 μl of overnight culture to 5 ml supplemented LB. Once inoculated, the pre-cultures were incubated for 2 h (37°C, 250 rpm) with loosened caps, to obtain growing cells. Next, co-evolution experiment cultures were set up in 96-well plates as follows. To avoid evaporation issues, the 36 wells around the edges of the plate were not used, and filled with 150 μl supplemented LB to serve as blanks/media controls. To the remaining 60 wells, in a randomised fashion (see Data Availability), a defined combination of bacteria, phage, and/or antibiotics were added (150 μl culture in total; see Fig 6) (n = 6 per combination). To each well, 130 μl of either of the 6 bacterial pre-cultures was added. Phages were added at an approximate multiplicity of infection (MOI) of approximately 0.25 to 0.3 (~3*107 CFU of E. coli C and ~7.5*106 to 9*106 PFU of ΦX174), and antibiotics were added at concentrations of 1 μg/ml or 2 μg/ml (Fig 6A). The plate was incubated in a microplate reader (Agilent BioTek Epoch 2 Microplate Spectrophotometer) for 24 h (37°C, 365 rpm double orbital shaking). Growth was measured as absorbance at 600 nm (OD600) every 5 min.
Day 2.
Approximately 3 μl (2%) of grown culture (from day 1) was transferred to 147 μl fresh supplemented LB in a new 96-well plate, with the same antibiotic conditions as before. Phages were not added separately (aside from the phages present in the 3 μl transfer). The plate was incubated under the same conditions for 24 h.
Statistical testing
T tests and Wilcoxon rank sum tests were used to determine whether there is a statistically significant difference in the mean concentration of antibiotic at which the growth of rough and deep rough E. coli C mutants is impeded (Fig 2B). T tests were performed using the stats package of R (R version 4.2.0, RStudio version 2023.12.1) [72,73]. Wilcoxon rank sum tests, with an exact solution for tied data sets, were performed using the EDISON WMW calculator [77]. Chi-squared tests for the goodness-of-fit of models (Table 2) were performed using the stats package of R (R version 4.2.0, RStudio version 2023.12.1) [72,73]. For the same, observed distributions of mutations by gene and by LPS type were compared with the distributions predicted by each model. In the 2-day coevolution experiment, environments were compared using the Tukey’s Honest Significant Difference (HSD) test combined with ANOVA (Fig 6B and 6C). An ANOVA model was fitted to the area under the bacterial growth curves in each environment, followed by pairwise comparisons of the different environments using the Tukey’s HSD test, both using the stats package of R (R version 4.2.0, RStudio version 2023.12.1) [72,73]. Growth on the different days was compared separately.
Data visualisation
Heatmaps (Fig 2) and histograms (Figs 3B, 3C, 4B, 5, and Figs S2 and S3 in S2 Text) were generated using Microsoft Excel (version 16.75.2). Schematic drawings (Figs 1, 2, and 3A) were created using BioRender.com. The genomic distribution of mutation loci (Fig 4A) and boxplot of antibiotic susceptibilities (Fig 2B) were visualised using R (RStudio version 2022.02.1) [72,73].
Supporting information
S1 Text. Note A. Explanation of differences in antibiotic susceptibility profiles between isogenic bacterial strains in Fig 2.
Note B. Parallel evolution in the fluctuation test. Note C. Predicted mutations from the fluctuation test that occur in genes not known to be related to LPS biosynthesis, assembly, or regulation.
https://doi.org/10.1371/journal.pbio.3002952.s001
(DOCX)
S2 Text. Fig S1. Raw mean greyscale values measured for strains grown in the presence of chloramphenicol.
The figure shows mean greyscale values for growth of different E. coli C strains combined with different chloramphenicol concentrations. There are between 1 and 5 measurements for each combination shown on the x-axis. Each measurement is the mean greyscale value across a region of 1,157.85 mm2 in a photo of an LB plate inoculated with an E. coli C strain (see Methods). The black horizontal line at a greyscale value of 37 indicates the mode of the mean background greyscale value of blank LB plates without addition of bacteria. The green horizontal line shows the strict growth cut-off of 8 (37+8) and the red horizontal line shows the lenient growth cut-off of 12 (37+12). The colours of the datapoints indicate whether the strain is predicted to possess a rough or a deep rough LPS phenotype. The data underlying this figure (greyscale values of replicates and raw images) can be found in https://doi.org/10.17617/3.PIDVUT. Fig S2. Raw mean greyscale values measured for strains grown in the presence of gentamicin. For details, see S1 Fig. Fig S3. Mean greyscale values of strains grown in the presence of chloramphenicol (A) or gentamicin (B), clustered by whether the strain is rough or deep rough. The figure shows the mean greyscale values for the growth of E. coli C at different antibiotic concentrations on LB plates for all strains shown in Fig 2. Each measurement is the mean greyscale value across a region of 1,157.85 mm2 in a photo of an LB plate inoculated with an E. coli C strain. The black horizontal line at a greyscale value of 37 indicates the mode of the mean background greyscale value, that is, of blank LB plates without addition of bacteria. The green horizontal line shows the strict growth cut-off of 8 (37+8) and the red horizontal line shows the lenient growth cut-off of 12 (37+12). The colours of the datapoints indicate whether the strain is predicted to possess a rough or a deep rough LPS phenotype. The data underlying this figure (greyscale values of replicates and raw images) can be found in https://doi.org/10.17617/3.PIDVUT. Fig S4. Types of mutations identified across phage-resistant E. coli C strains isolated from the fluctuation test. Bars show all predicted mutations grouped by mutational class (as % of total mutations “n” identified in each of the 5 plating environments sampled). If an isolate had 2 mutations, they were classified and counted separately. Insertions and deletions are further separated into 4 categories: (i) transposon insertion, where the inserted bases are a transposable element, (ii) duplication, where the inserted bases match the sequence preceding the mutated locus (up to 1.3 kbp), (iii) large deletion, where a region of more than 100 bp is deleted, and (iv) small indels, other insertions and deletions of S4 Table. Fig S5. Comparison of model 3 predictions with observed phage-resistance rates. (A) Phage-resistance rates for phage–antibiotic combinations, relative to the phage-resistance rate in the ΦX174-only environment. Observed rates (black) are only qualitatively in line with the predicted rates from model 1 (gene length model, dark red; see Table 1). Rates predicted by model 2 (dark yellow; derived in S6 Table) are within 95% confidence intervals for ΦX174+gentamicin environments and are 2% away from the 95% confidence interval in ΦX174+chloramphenicol (2 μg/ml) environment. Phage-resistance rates for model 3 (blue, S7 Table) remain largely unchanged (relative to those for model 2), except for the ΦX174+gentamicin (2 μg/ml) environment (where the prediction becomes worse). (B) LPS phenotype distribution in phage-resistant E. coli C mutants isolated in the fluctuation test. Graphs show proportion of mutants with a certain LPS phenotype (values in S5 Table). Model 1 predictions (dark red) are qualitatively in line with predictions. In model 2 (dark yellow), LPS phenotype distribution in the ΦX174-only environment is now in line with the observations. Model 3 predictions (blue) improve slightly for ΦX174+chloramphenicol (2 μg/ml) and ΦX174+gentamicin (2 μg/ml) environments. (C–F) Gene-wise distribution compared to model 3 predictions (and model 2 in C and D). In ΦX174+chloramphenicol (1 μg/ml), mutations are predicted in waaF, gmhB, gmhA, and rfaH, but none are observed. Similarly, in ΦX174+gentamicin (2 μg/ml), mutations in gmhB, gmhA, hldE are predicted but not observed. Compared to model 2, only predictions at ΦX174+chloramphenicol (2 μg/ml) (waaP, galU) and ΦX174+gentamicin (2 μg/ml) (gmhB, gmhA, hldE) change in model 3. Error bars show the standard deviation for each LPS phenotype (B) or each gene (C–F) from 100 bootstrapping iterations (resampling with replacement, see Methods). The data underlying this figure can be found in S4–S7 Tables, which include raw mutation data as well as summarised values.
https://doi.org/10.1371/journal.pbio.3002952.s002
(DOCX)
S2 Table. Resistance rates observed in the fluctuation test.
**Nt is the total number of cells in the culture. #ε is the fraction of cells plated. §We counted phage-resistant colonies from 50 biological replicates for each plating environment, which were used to calculate phage-resistance rates and 95% confidence intervals (with rSalvador; see Methods). $The likelihood-ratio test statistic from rSalvador was used to determine whether each calculated phage-resistance rate is significantly different to that of the ΦX174-only plating environment. *Mutation rates and significance intervals for GM 4 μg/ml were calculated without counting plates that contained too many colonies. †Resistance rate relative to the resistance rate in the ΦX17-only plating environment, calculated for combination plating environments with chloramphenicol (CL) or gentamicin (GM). Raw data (colony counts) are provided in S3 Table. Source data for Fig 3.
https://doi.org/10.1371/journal.pbio.3002952.s004
(XLSX)
S3 Table. Colony counts from the fluctuation test.
**Phage-resistant colonies were counted from 50 biological replicates for each fluctuation test plating environment. The resulting colony counts were used to calculate phage-resistance rates and 95% confidence intervals (see Methods). *“Burst”: some plates contained areas with large numbers of tiny colonies (i.e., uncountable). #“invalid”: Plate discarded due to contamination. Source data for Fig 3.
https://doi.org/10.1371/journal.pbio.3002952.s005
(XLSX)
S4 Table. Details of mutations predicted in the phage-resistant E. coli C strains isolated from the fluctuation test.
$DUP indicates a duplication event. #“2,611,543, +A”: indicates an insertion of “A” after nucleotide 2,611,543. **“2,611,295–2,611,303, INS (2,783,815–2,784,945 INV)”: indicates positions 2,783,815–2,784,945 were inserted (inverted) at position 2,611,303. The inserted nucleotides are flanked by 2,611,295–2,611,303 on both sides (i.e., 2,611,295–2,611,303 are duplicated). §E187fs_Ter#192 indicates a frameshift starting at E187, with a stop codon (Ter) generated at codon #192. F366mod_Ter#383 indicates protein sequence modified, e.g., due to a large insertion/duplication starting from F366, and (premature) stop codon (Ter) generated at codon #383. Difference between frameshift (fs) and modification (mod) is whether the original sequence of the gene is used as coding region with only a frameshift, or the new inserted sequence is used until the stop codon. ||Predicted LPS phenotypes for the mutants and the mutations in their genome. *Where multiple LPS genes carry mutations, the star indicates the gene predicted to cause the most dramatic LPS phenotype change. ^Column indicates whether the mutated gene is related to LPS biosynthesis, assembly, or regulation. †Even though the amino acid is unchanged, the codon affected is the start codon ATG, hence the initiation of translation is expected to be inhibited. See Fig S4 in S2 Text for a summary of mutational classes.
https://doi.org/10.1371/journal.pbio.3002952.s006
(XLSX)
S6 Table. Details of Model 2 used to predict phage-resistance rates.
See Methods for model 2 assumptions. **New genes. Highlighted in green: 2 genes in which 0 mutations were detected among isolates from the ΦX17-only plating environment, due to which a pseudo-count or pseudo-weight of 1 was added to all genes (including these 2 genes).
https://doi.org/10.1371/journal.pbio.3002952.s008
(XLSX)
Acknowledgments
The authors thank the Barrick Lab for their fluctuation test protocols, Prof. Qi Zheng for his help with rSalvador, Dr. Carsten Fortmann-Grote for his help with image analysis, and all members of the Microbial Population Biology department who helped with experiments, especially Daniel Martens, Dr. Elisa Brambilla, and Lena Zeller.
References
- 1.
World Health Organization. Antimicrobial resistance: global report on surveillance [Internet]. World Health Organization; 2014 [cited 2021 Oct 18]. xxii, 232 p. Available from: https://apps.who.int/iris/handle/10665/112642. - 2.
Czaplewski L, Bax R, Clokie M, Dawson M, Fairhead H, Fischetti VA, et al. Alternatives to antibiotics—a pipeline portfolio review. Lancet Infect Dis. 2016 Feb 1;16(2):239–251. pmid:26795692 - 3.
Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob Agents Chemother. 2017 Oct;61(10):e00954–e00917. pmid:28807909 - 4.
Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health. 2018 Jan 1;2018(1):60–66. pmid:29588855 - 5.
Abedon ST. Use of phage therapy to treat long-standing, persistent, or chronic bacterial infections. Adv Drug Deliv Rev. 2019 May;145:18–39. pmid:31708017 - 6.
Pirnay JP, Ferry T, Resch G. Recent progress toward the implementation of phage therapy in Western medicine. FEMS Microbiol Rev. 2022 Jan 18;46(1):fuab040. pmid:34289033 - 7.
Oechslin F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses. 2018 Jun 30;10(7):351. pmid:29966329 - 8.
Coulter LB, McLean RJC, Rohde RE, Aron GM. Effect of Bacteriophage Infection in Combination with Tobramycin on the Emergence of Resistance in Escherichia coli and Pseudomonas aeruginosa Biofilms. Viruses. 2014 Oct;6(10):3778–86. pmid:25285538 - 9.
Torres-Barceló C, Arias-Sánchez FI, Vasse M, Ramsayer J, Kaltz O, Hochberg ME. A Window of Opportunity to Control the Bacterial Pathogen Pseudomonas aeruginosa Combining Antibiotics and Phages. PLoS ONE. 2014 Sep 26;9(9):e106628. pmid:25259735 - 10.
Chaudhry WN, Concepción-Acevedo J, Park T, Andleeb S, Bull JJ, Levin BR. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE. 2017 Jan 11;12(1):e0168615. pmid:28076361 - 11.
Kebriaei R, Lev KL, Shah RM, Stamper KC, Holger DJ, Morrisette T, et al. Eradication of Biofilm-Mediated Methicillin-Resistant Staphylococcus aureus Infections In Vitro: Bacteriophage-Antibiotic Combination. Microbiol Spectr. 2022 Mar 29;10(2):e00411–e00422. pmid:35348366 - 12.
Valério N, Oliveira C, Jesus V, Branco T, Pereira C, Moreirinha C, et al. Effects of single and combined use of bacteriophages and antibiotics to inactivate Escherichia coli. Virus Res. 2017 Aug;240:8–17. pmid:28746884 - 13.
Cairns J, Becks L, Jalasvuori M, Hiltunen T. Sublethal streptomycin concentrations and lytic bacteriophage together promote resistance evolution. Philos Trans R Soc B Biol Sci. 2017 Jan 19;372(1712):20160040. pmid:27920385 - 14.
Hancock RE, Reeves P. Lipopolysaccharide-deficient, bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):98–108. pmid:776951 - 15.
Mutalik VK, Adler BA, Rishi HS, Piya D, Zhong C, Koskella B, et al. High-throughput mapping of the phage resistance landscape in E. coli. PLoS Biol. 2020 Oct 13;18(10):e3000877. pmid:33048924 - 16.
Romeyer Dherbey J, Parab L, Gallie J, Bertels F. Stepwise Evolution of E. coli C and ΦX174 Reveals Unexpected Lipopolysaccharide (LPS) Diversity. Mol Biol Evol. 2023 Jul 1;40(7):msad154. - 17.
Bertozzi Silva J, Storms Z, Sauvageau D. Host receptors for bacteriophage adsorption. FEMS Microbiol Lett [Internet]. 2016 Feb 1 [cited 2021 Nov 30];363(4). pmid:26755501 - 18.
Bio-Conversion Databank Foundation [Internet]. Bio-Conversion Databank Foundation Portal. 2021 [cited 2023 Mar 3]. Available from: https://portal.bio-conversion.org/. - 19.
Frirdich E, Whitfield C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J Endotoxin Res. 2005;11(3):133–144. pmid:15949142 - 20.
Yethon JA, Heinrichs DE, Monteiro MA, Perry MB, Whitfield C. Involvement of waaY, waaQ, and waaP in the modification of Escherichia coli lipopolysaccharide and their role in the formation of a stable outer membrane. J Biol Chem. 1998 Oct 9;273(41):26310–26316. pmid:9756860 - 21.
Nikaido H, Vaara M. Molecular Basis of Bacterial Outer Membrane Permeability. Microbiol Rev. 1985;49:32. pmid:2580220 - 22.
Michel A, Clermont O, Denamur E, Tenaillon O. Bacteriophage PhiX174’s Ecological Niche and the Flexibility of Its Escherichia coli Lipopolysaccharide Receptor. Appl Environ Microbiol. 2010 Nov 1;76(21):7310–7313. pmid:20833781 - 23.
Sinsheimer RL. Purification and properties of bacteriophage φX174. J Mol Biol. 1959 Apr;1(1):37–IN5. - 24.
Sanger F, Air GM, Barrell BG, Brown NL, Coulson AR, Fiddes JC, et al. Nucleotide sequence of bacteriophage φX174 DNA. Nature. 1977 Feb;265(5596):687–695. - 25.
Smith HO, Hutchison CA, Pfannkoch C, Venter JC. Generating a synthetic genome by whole genome assembly: φX174 bacteriophage from synthetic oligonucleotides. Proc Natl Acad Sci U S A. 2003 Dec 23;100(26):15440–15445. - 26.
Ebbensgaard A, Mordhorst H, Aarestrup FM, Hansen EB. The Role of Outer Membrane Proteins and Lipopolysaccharides for the Sensitivity of Escherichia coli to Antimicrobial Peptides. Front Microbiol. 2018 Sep 7;9:2153. pmid:30245684 - 27.
Burmeister AR, Fortier A, Roush C, Lessing AJ, Bender RG, Barahman R, et al. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc Natl Acad Sci U S A. 2020 May 26;117(21):11207–11216. pmid:32424102 - 28.
Nikaido H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol Mol Biol Rev. 2003 Dec;67(4):593–656. pmid:14665678 - 29.
El’Garch F, Jeannot K, Hocquet D, Llanes-Barakat C, Plésiat P. Cumulative Effects of Several Nonenzymatic Mechanisms on the Resistance of Pseudomonas aeruginosa to Aminoglycosides. Antimicrob Agents Chemother. 2007 Mar;51(3):1016–1021. pmid:17194835 - 30.
Dötsch A, Becker T, Pommerenke C, Magnowska Z, Jänsch L, Häussler S. Genomewide Identification of Genetic Determinants of Antimicrobial Drug Resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009 Jun;53(6):2522–2531. pmid:19332674 - 31.
Klein G, Raina S. Regulated Assembly of LPS, Its Structural Alterations and Cellular Response to LPS Defects. Int J Mol Sci. 2019 Jan 16;20(2):356. pmid:30654491 - 32.
Yethon JA, Vinogradov E, Perry MB, Whitfield C. Mutation of the lipopolysaccharide core glycosyltransferase encoded by waaG destabilizes the outer membrane of Escherichia coli by interfering with core phosphorylation. J Bacteriol. 2000 Oct;182(19):5620–5623. pmid:10986272 - 33.
Lin J, Zhou D, Steitz TA, Polikanov YS, Gagnon MG. Ribosome-Targeting Antibiotics: Modes of Action, Mechanisms of Resistance, and Implications for Drug Design. Annu Rev Biochem. 2018 Jun 20;87:451–478. pmid:29570352 - 34.
Ibacache-Quiroga C, Oliveros JC, Couce A, Blázquez J. Parallel Evolution of High-Level Aminoglycoside Resistance in Escherichia coli Under Low and High Mutation Supply Rates. Front Microbiol. 2018 Mar 19;9:427. pmid:29615988 - 35.
Frost I, Smith WPJ, Mitri S, Millan AS, Davit Y, Osborne JM, et al. Cooperation, competition and antibiotic resistance in bacterial colonies. ISME J. 2018 Jun;12(6):1582–1593. pmid:29563570 - 36.
Mahrt N, Tietze A, Künzel S, Franzenburg S, Barbosa C, Jansen G, et al. Bottleneck size and selection level reproducibly impact evolution of antibiotic resistance. Nat Ecol Evol. 2021 Sep;5(9):1233–1242. pmid:34312522 - 37.
Farr AD, Pesce D, Das SG, Zwart MP, de Visser JAGM. The Fitness of Beta-Lactamase Mutants Depends Nonlinearly on Resistance Level at Sublethal Antibiotic Concentrations. MBio. 2023 Apr 27;14(3):e00098–e00023. pmid:37129484 - 38.
Aduru SV, Szenkiel K, Rahman A, Ahmad M, Fabozzi M, Smith RP, et al. Sub-inhibitory antibiotic treatment selects for enhanced metabolic efficiency. Microbiol Spectr. 2024 Jan 16;0(0):e03241–e03223. pmid:38226801 - 39.
Luria SE, Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov 1;28(6):491–511. pmid:17247100 - 40.
Rosche WA, Foster PL. Determining Mutation Rates in Bacterial Populations. Methods San Diego Calif. 2000 Jan;20(1):4–17. pmid:10610800 - 41.
Zheng Q. rSalvador: An R Package for the Fluctuation Experiment. G3. 2017 Dec 1;7(12):3849–56. pmid:29084818 - 42.
Pradel E, Schnaitman CA. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991 Oct;173(20):6428–6431. pmid:1655711 - 43.
Lind PA, Libby E, Herzog J, Rainey PB. Predicting mutational routes to new adaptive phenotypes. Wittkopp PJ, editor. Elife. 2019 Jan 8;8:e38822. pmid:30616716 - 44.
Horton JS, Flanagan LM, Jackson RW, Priest NK, Taylor TB. A mutational hotspot that determines highly repeatable evolution can be built and broken by silent genetic changes. Nat Commun. 2021 Oct 19;12(1):6092. pmid:34667151 - 45.
Comeau AM, Tétart F, Trojet SN, Prère MF, Krisch HM. Phage-Antibiotic Synergy (PAS): β-Lactam and Quinolone Antibiotics Stimulate Virulent Phage Growth. PloS One. 2007 Aug 29;2(8):e799. - 46.
Gu Liu C, Green SI, Min L, Clark JR, Salazar KC, Terwilliger AL, et al. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. MBio. 2020 Aug 4;11(4):e01462–e01420. pmid:32753497 - 47.
Baym M, Stone LK, Kishony R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science. 2016 Jan 1;351(6268):aad3292. pmid:26722002 - 48.
Barbosa C, Trebosc V, Kemmer C, Rosenstiel P, Beardmore R, Schulenburg H, et al. Alternative Evolutionary Paths to Bacterial Antibiotic Resistance Cause Distinct Collateral Effects. Mol Biol Evol. 2017 Sep 1;34(9):2229–2244. pmid:28541480 - 49.
Coates J, Park BR, Le D, Şimşek E, Chaudhry W, Kim M. Antibiotic-induced population fluctuations and stochastic clearance of bacteria. Walczak AM, editor. Elife. 2018 Mar 6;7:e32976. pmid:29508699 - 50.
Akiyama T, Kim M. Stochastic response of bacterial cells to antibiotics: its mechanisms and implications for population and evolutionary dynamics. Curr Opin Microbiol. 2021;63:104–108. pmid:34325154 - 51.
Hadas H, Einav M, Fishov I, Zaritsky A. Bacteriophage T4 Development Depends on the Physiology of its Host Escherichia Coli. Microbiology. 1997;143(1):179–185. pmid:9025292 - 52.
Moxon ER, Rainey PB, Nowak MA, Lenski RE. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol. 1994 Jan 1;4(1):24–33. pmid:7922307 - 53.
Bertels F, Metzner KJ, Regoes R. Convergent evolution as an indicator for selection during acute HIV-1 infection. Peer Community J. 2021 Nov 23;1:e4. - 54.
Wang Z, Wang J, Ren G, Li Y, Wang X. Influence of Core Oligosaccharide of Lipopolysaccharide to Outer Membrane Behavior of Escherichia coli. Mar Drugs. 2015 May 27;13(6):3325–3339. pmid:26023839 - 55.
Burns JL, Hedin LA, Lien DM. Chloramphenicol resistance in Pseudomonas cepacia because of decreased permeability. Antimicrob Agents Chemother. 1989 Feb;33(2):136–141. pmid:2719457 - 56.
Recht MI, Puglisi JD. Aminoglycoside Resistance with Homogeneous and Heterogeneous Populations of Antibiotic-Resistant Ribosomes. Antimicrob Agents Chemother. 2001 Sep;45(9):2414–2419. pmid:11502507 - 57.
Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol. 2005 Aug;57(4):1064–1073. pmid:16091044 - 58.
Chang V, Chen LY, Wang A, Yuan X. The Effect of Lipopolysaccharide Core Structure Defects on Transformation Efficiency in Isogenic Escherichia coli BW25113 rfaG, rfaP, and rfaC Mutants. 2010;14:7. - 59.
Pagnout C, Sohm B, Razafitianamaharavo A, Caillet C, Offroy M, Leduc M, et al. Pleiotropic effects of rfa-gene mutations on Escherichia coli envelope properties. Sci Rep. 2019 Dec;9(1):9696. pmid:31273247 - 60.
Romeyer Dherbey J, Bertels F. The untapped potential of phage model systems as therapeutic agents. Virus Evol. 2024 Jan 1;10(1):veae007. pmid:38361821 - 61.
Yehl K, Lemire S, Yang AC, Ando H, Mimee M, Torres MDT, et al. Engineering Phage Host-Range and Suppressing Bacterial Resistance through Phage Tail Fiber Mutagenesis. Cell. 2019 Oct 3;179(2):459–469.e9. pmid:31585083 - 62.
Suzuki M, Kaneko-Tanaka Y, Azegami M. Transfection of non-host bacterial spheroplasts with bacteriophage ΦX174 DNA. Nature. 1974 Nov;252(5481):319–321. - 63.
Mayr UB, Walcher P, Azimpour C, Riedmann E, Haller C, Lubitz W. Bacterial ghosts as antigen delivery vehicles. Adv Drug Deliv Rev. 2005 Jun 17;57(9):1381–1391. pmid:15878634 - 64.
Orta AK, Riera N, Li YE, Tanaka S, Yun HG, Klaic L, et al. The mechanism of the phage-encoded protein antibiotic from ΦX174. Science. 2023 Jul 14;381(6654):eadg9091. - 65.
Burns SM, Hull SI. Comparison of Loss of Serum Resistance by Defined Lipopolysaccharide Mutants and an Acapsular Mutant of Uropathogenic Escherichia coli O75:K5. Infect Immun. 1998 Sep;66(9):4244–4253. pmid:9712774 - 66.
Raetz CRH, Whitfield C. Lipopolysaccharide Endotoxins. Annu Rev Biochem. 2002;71:635–700. pmid:12045108 - 67.
Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol IJMM. 2007 Sep;297(5):277–295. pmid:17466590 - 68.
Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7(3):167–202. pmid:11581570 - 69.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Vol. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2019. - 70.
Allan C, Burel JM, Moore J, Blackburn C, Linkert M, Loynton S, et al. OMERO: flexible, model-driven data management for experimental biology. Nat Methods. 2012 Mar;9(3):245–253. pmid:22373911 - 71.
Foster PL. Methods for Determining Spontaneous Mutation Rates. Methods Enzymol. 2006;409:195–213. pmid:16793403 - 72.
RStudio Team. RStudio: Integrated Development Environment for R [Internet]. Boston, MA: RStudio, Inc.; 2019. Available from: http://www.rstudio.com/. - 73.
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org/. - 74.
Simon A. FastQC A Quality Control tool for High Throughput Sequence Data [Internet]. 2010 [cited 2023 Apr 19]. Available from: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/. - 75.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014 Aug 1;30(15):2114–2120. pmid:24695404 - 76.
Deatherage DE, Barrick JE. Identification of mutations in laboratory evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol Clifton NJ. 2014;1151:165–188. pmid:24838886 - 77.
Marx A, Backes C, Meese E, Lenhof HP, Keller A. EDISON-WMW: Exact Dynamic Programing Solution of the Wilcoxon–Mann–Whitney Test. Genom Proteom Bioinform. 2016 Feb;14(1):55–61. pmid:26829645
ADVERTISEMENT:
Hai, sobat pencinta slots pernahkah mendengar istilah “raja slot? jika tidak, bersiaplah jatuh hati dengan konsep ini. raja slot merupakan mesin slots yang selalu memberi win. Yup, slot-slot ini bisa dikatakan sebagai jagoannya tuk bawa pulang cuan. any way, gimana sih caranya nemuin slot gaco yang tepat? Tenang Bro, kita beri tenang saja di sini
Game terpercaya waktu ini hanya satu di Indonesia hanya di pasti menyediakan imbal hasil terbaik
SEGERA hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia