Citation: Koenig L, He BJ (2025) Spontaneous slow cortical potentials and brain oscillations independently influence conscious visual perception. PLoS Biol 23(1):
e3002964.
https://doi.org/10.1371/journal.pbio.3002964
Academic Editor: Christopher Pack, McGill University, CANADA
Received: May 22, 2024; Accepted: December 3, 2024; Published: January 16, 2025
Copyright: © 2025 Koenig, He. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All code used to run the analysis are available at https://doi.org/10.5281/zenodo.14236766. All the data relevant to plotting the figures are available at https://doi.org/10.5281/zenodo.14291607.
Funding: This work was supported by a U.S. National Institutes of Health grant (R01EY032085, to BJH) and an Irma T. Hirschl Career Scientist Award (to BJH). The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations:
AUC,
area under the curve; AUROC,
area under the ROC; BW,
bandwidth; CD,
Coordinate Descent; CF,
center frequency; d.v.,
decision variable; EEG,
electroencephalography; ICA,
independent component analysis; LC,
locus coeruleus; LC-NE,
locus coeruleus-norepinephrine; MEG,
magnetoencephalography; ROC,
receiver-operator curve; SCP,
slow cortical potential; SVM,
support vector machine
Introduction
A key characteristic of perception is its variability over time: we might notice a crack in a windshield one day, finding it distracting and hard to ignore, but on the next day, fail to notice the crack entirely, as it fades into the background as we focus on driving. This variability shows that conscious perception is not determined solely by the incoming sensory information; instead, it is molded by additional neural mechanisms intrinsic in the brain that vary over time [1,2].
A long-standing line of work using human fMRI has revealed that the constantly fluctuating, large-scale spontaneous activity of the brain plays a crucial role in shaping conscious perception in the visual [3], auditory [4], and somatosensory [5] modalities. However, the underlying dynamical mechanisms remain unclear. Using electroencephalography (EEG) and magnetoencephalography (MEG), a separate line of studies has discovered 2 distinct electrophysiological signatures of pre-stimulus spontaneous activity that influence conscious perception: power [6,7] and phase [8,9] of brain oscillations in the alpha (8 to 12 Hz) and beta (13 to 30 Hz) frequency ranges, on the one hand, and large-scale activity patterns of aperiodic activity in the slow cortical potential (SCP, 10–12]. However, currently, a key unanswered question is whether brain oscillations and SCPs act on perception independently or through a common set of mechanisms. Addressing this question would reveal whether there is a common final pathway for diverse spontaneous electrophysiological phenomena to exert their influences on perception or, alternatively, whether there may be a multitude of channels through which spontaneous neural dynamics shape perception.
In this study, we focus on the power of alpha and beta oscillations and investigate whether their influences on conscious visual perception share mechanisms with SCP or rather operate independently. Alpha oscillations have been extensively studied in the context of visual perception. In occipital regions, pre-stimulus alpha power predicts whether a near-threshold stimulus will be perceived or missed [6,13–17]. These findings fit well with the interpretation that alpha oscillations reflect fluctuating cortical excitability in sensory regions [18–20]. Importantly, these findings were also supported by recent work that separated the alpha oscillations from the 1/f power spectrum [21]. Alpha oscillations might also influence perception through attentional mechanisms, since fluctuations in alpha power can correlate with shifts in attention [6,22–24]. In addition, neuromodulatory systems influencing arousal, with consequences on sensory and perceptual processing [25,26], may operate through changes in alpha oscillations [27–30].
Although less extensively studied, beta oscillations have also been suggested to influence conscious perception, specifically via changes in visuospatial attentional engagement. For instance, modulation of selective attention was shown to be associated with long-range phase synchrony in the beta band within the frontoparietal attentional network, resulting in enhanced target processing and suppressed nontarget processing in a visual task [31]. Similarly, higher parietal beta power was found to predict the accuracy of perceptual choices [32]. Further, repetitive TMS over the frontal eye field in the beta range facilitated conscious visual perception in a near-threshold task [33]. Taken together, these studies show that alpha and beta oscillations may influence conscious perception through a variety of neural mechanisms.
In addition to oscillatory activity, aperiodic SCPs have also been shown to powerfully modulate the conscious perception and recognition of visual stimuli. In visual tasks, the phase of spontaneous SCP shifts prior to the onset of a threshold-level stimulus influences the likelihood of detection [34]. Recent studies have shown that the pre-stimulus large-scale activity pattern of the SCP predicts whether a low-level visual stimulus will be seen [10], whether a high-level visual stimulus will be recognized [12], and which perceptual content will reach awareness in a bistable perception task [35]. Additionally, SCPs can correlate with pupil-linked arousal [12], which influences sensory encoding and perceptual processing [30,36–38]. Importantly, both pre-stimulus SCP and pre-stimulus alpha power have been shown to influence the criterion in conscious perception tasks [6,12], further underscoring the question of whether they might act on perception through shared mechanisms.
Despite strong evidence suggesting the involvement of both types of spontaneous activity (brain oscillations and aperiodic SCP) in conscious perception, it is currently unknown whether they interact with each other or rather act independently to modulate visual perception. The current study aimed to address this question. Further, we aimed to elucidate these neurophysiological signals’ relations to pupil size, a noninvasive proxy of moment-to-moment arousal fluctuations [36,39]. We analyzed 2 previously collected MEG data sets [10,12], in which participants performed a threshold-level visual perception task involving either low-level (Gabor patches) or high-level (images containing objects, faces, houses, or animals) visual stimuli. We extracted perceptually relevant measures of pre-stimulus alpha power, beta power and SCPs, and examined whether their influences on perceptual outcome had shared variances. The results reveal that brain oscillations and SCPs influence conscious visual perception through independent mechanisms and that their perceptual influences have distinct relations to arousal-mediated mechanisms.
Results
Paradigm and behavior
To investigate the influence of pre-stimulus neural activity on conscious visual perception, we analyzed 2 previously collected MEG data sets [10,12]. In these tasks, subjects were presented with a visual stimulus, specifically, a low-level Gabor stimulus (which was either left- or right-tilting) in the “low-level data set” and a high-level stimulus (which was either an animal, a face, an object, or a house) in the “high-level data set.” The stimuli were titrated to the individual subject’s perceptual threshold. Subjects were instructed to indicate whether they had consciously detected the stimulus (low-level data set) or consciously recognized a meaningful content in the stimulus (high-level data set). Previous work using these 2 data sets has shown that pre-stimulus large-scale activity patterns in the SCP, in a 2-s window preceding stimulus onset, strongly influence whether a threshold-level low-level or high-level stimulus enters conscious awareness [10,12]. Here, we first show that pre-stimulus alpha and beta oscillatory powers in the same time window also influence conscious perception. We then investigate their relationships with pre-stimulus SCP activity’s influence on perception (Fig 1G).
Fig 1. Paradigm, behavior, and overall methods.
(A) Paradigm timeline for the low-level data set. Each trial began with a blank screen, after which a brief Gabor patch was presented for a duration determined by the participant’s individual detection threshold. Subjects were asked to report the orientation of the stimulus and their detection experience. MEG activity in a 1.6 s window prior to stimulus onset (indicated by horizontal red bar) was used in further analyses. (B) Percentage of trials in which subjects reported detecting the stimulus, in real and catch trials. The dashed line represents the intended threshold-level detection rate (left panel). Percentage of trials in which subjects reported the correct orientation of the stimulus, for seen (Y, pink) and unseen (N, blue) trials. The dashed line represents the chance level of 50% for orientation discrimination (right panel). (C) Paradigm timeline for the high-level data set. Each trial began with a blank screen, after which a brief object stimulus was presented for 66 ms at a contrast determined by the participant’s individual recognition threshold. Subjects were asked to report the category of the stimulus and their recognition experience. MEG was analyzed in a 1.6 s window prior to stimulus onset. (D) Percentage of trials in which subjects reported recognizing the stimulus for real and scrambled images. The dashed line indicates the intended threshold-level recognition rate for real images (left panel). Percentage of correctly categorized images, when stimuli were reported as recognized (pink) and unrecognized (blue), for real and scrambled images. The dashed line represents the chance level of 25% for categorization (right panel). In all box plots, the center line depicts the median and the edges indicate the quartiles. Stars above boxplots report paired tests, whereas stars below the dashed line report one-sample tests against a chance level of 25%; * p p ≤ 0.01, *** p ≤ 0.001. (E) Schematic representation of decoding from SCP activity. For each subject and pre-stimulus temporal window, the SCP activity from all seen or recognized (Y, pink) and unseen or unrecognized (N, blue) trials was projected onto a high-dimensional space (where each dimension corresponds to each MEG sensor, here represented for 2 hypothetical sensors). Multivariate decoders were trained to find the best hyperplane to separate trials based on perceptual outcomes. We used the distance of each trial from this decision hyperplane as the “SCP decision variable”, or SCP d.v., plotted here with a dashed black line. (F) In the left panel, we obtained the power spectrum across the pre-stimulus temporal interval for each subject and extracted the oscillatory peaks in the alpha and beta ranges. We removed the aperiodic fit (dashed blue line) from the full power spectrum (“full model fit”, red line) and computed the area under the curve (green shaded area) in alpha and beta bands to obtain their respective powers. This figure was adapted from the fooof toolbox [47]. In the right panel, we depict the topographic plots of the activity obtained by subtracting activity in unseen or unrecognized trials from the activity in seen or recognized trials. Wilcoxon signed-rank tests were then performed at each sensor. Adjacent sensors that showed significant differences with the same sign were clustered together. We determined which clusters were significant through cluster-based permutation. (G) The analyses will investigate whether perceptually relevant spontaneous aperiodic activity, specifically SCP d.v., correlates with perceptually relevant spontaneous oscillatory activity, specifically alpha power and beta power. The data underlying this figure can be found as Fig 1_Data at https://doi.org/10.5281/zenodo.14291607. d.v., decision variable; MEG, magnetoencephalography; SCP, slow cortical potential.
We first describe the behavioral patterns in both data sets. In the low-level data set, subjects were shown a brief-duration (33 to 66 ms), low-contrast Gabor patch and asked to indicate whether they had consciously seen the stimulus, and what its tilt was (Fig 1A). On each trial, the orientation of the Gabor patch was randomly chosen between 45° and 135°, and subjects were asked to provide a guess about its orientation even if they did not subjectively perceive it (i.e., a two-alternative forced choice question). A small number of catch trials were also included, in which no stimulus was presented. On average, subjects reported seeing the stimulus in an average of 48.9 ± 4.4% (mean ± SEM) of “real” (i.e., stimulus present) trials—suggesting that the stimuli were indeed presented at subjective threshold—and in 0.09 ± 0.29% of catch trials (Fig 1B). When they reported seeing the stimulus in real trials, they correctly identified the orientation in 96.8 ± 1.3% of trials. When they reported not seeing the stimulus, they correctly identified the orientation in 62.0 ± 2.7% of trials, which was still significantly above the chance level of 50% (one-sample t test against chance level of 50%, p 40,41]. The paradigm and behavioral patterns in this data set were described in detail in previous papers [10,42].
In the high-level data set, subjects were shown a brief (66 ms), low-contrast object stimulus and asked to report whether they subjectively recognized a meaningful content in the image (i.e., recognition experience) and what the category of the image was (i.e., categorization report; see Fig 1C). The object image was randomly chosen from one of 4 categories on each trial. The object image set consisted of 20 unique images, with 5 exemplars in each category. In addition, in a small percentage of trials, a scrambled image was shown, created by phase scrambling one exemplar image from each category (Fig 1C, inset, bottom row), and the scrambled image was presented at the same contrast as its original counterpart. The phase scrambling procedure destroyed any meaningful content in the original image but preserved low-level image features that have different statistical properties across categories. Therefore, reporting subjectively recognizing a meaningful content in a scrambled image constituted a false alarm. On average, subjects reported recognizing 44.9 ± 3.5% of real images (consistent with the thresholding procedure which aimed for 50% recognition rate) and 17.2 ± 3.3% of scrambled images (Fig 1D). The categorization accuracy in real image trials was 86.5 ± 1.8% for recognized trials and 40.1 ± 1.8% for unrecognized trials, which were significantly different (paired t test, p p = 0.0015). Categorization accuracy was significantly above chance level in all conditions (one-sample t tests, all p 12].
In the following analyses, we investigate pre-stimulus brain activity predisposing the subject to have a conscious visual experience, as indicated by a “seen” response in the low-level data set and a “recognized” response in the high-level data set. Both because the previous literature has shown that the effects of interest (alpha/beta power and SCPs) predict criterion effects [6,10,12,43], which correspond to perceptual reports in both real and catch/scrambled trials, and to increase statistical power, the analyses use all trials in both data sets, including both real and catch/scrambled trials (catch trials accounted for approximately 5% of trials in the low-level data set; scrambled trials 16.7% of all trials in the high-level data set). As such, we focus on whether different pre-stimulus neural activities have shared influences on perceptual outcome, instead of dissociating pre-stimulus activity’s influence on sensitivity and criterion related to conscious perception as previous studies have done [6,12].
Whole-brain SCP activity patterns in the pre-stimulus period predict perceptual outcome
SCPs are the low-frequency (44]. To extract SCP activity, we first filtered MEG activity in the 0.05 to 5 Hz range [10]. Using this activity across the whole brain, we trained a classifier to discriminate between perceived and unperceived (i.e., seen versus unseen in the low-level data set, recognized versus unrecognized in the high-level data set) trials in the pre-stimulus interval spanning 1.7 to 0.1 s before stimulus onset. A simulation showed that at 0.1 s before stimulus onset, the data are unaffected by any temporal spearing of post-stimulus activity due to the frequency-domain filter applied (Fig A in S1 Text).
The decoder outputs a decision variable (d.v.), which is a continuously valued variable that measures the strength of evidence for each perceptual outcome, with positive values suggesting that the “seen/recognized” outcome is preferred and negative values suggesting that the “unseen/unrecognized” outcome is preferred (Fig 1E). Thus, for each trial, we obtained the SCP d.v., quantifying the strength of evidence for either perceptual outcome, at each time point within the pre-stimulus interval.
Consistent with the original publications showing successful pre-stimulus SCP decoding of perceptual outcome [10,12], the SCP d.v. timecourse averaged across trials and subjects was positive for perceived trials and negative for unperceived trials across the pre-stimulus intervals in both the low-level data set (Fig 2A) and the high-level data set (Fig 2D), suggesting that SCP contains significant predictive information about the outcome of conscious perception at the single-trial level.
Fig 2. Alpha power and SCP d.v. both influence perceptual outcome but do not covary.
(A) Timecourse of the mean SCP d.v. activity (n = 11) across the pre-stimulus interval for seen and unseen trials in the low-level data set. The dashed horizontal line represents an SCP d.v. of 0, indicating that the whole-brain SCP activity pattern does not distinguish between seen and unseen trials. The dotted vertical lines indicate the limits of the 4 temporal intervals considered during the analysis. The thick vertical red line indicates stimulus onset. Shaded areas indicate the paired sem. (B) Topographic plot of the alpha power cluster in the low-level data set (left). Timecourse of mean alpha power across the pre- and post-stimulus intervals for seen and unseen trials (right). The shaded gray area indicates the temporal interval in which this cluster was obtained. (C) Correlation between alpha power and SCP d.v. across single trials in the low-level data set. The top panel represents the boxplot of z-transformed correlation coefficients across subjects, tested against 0 with a Wilcoxon signed-rank test; n.s.: not significant. Dashed horizontal lines represent a null correlation. The bottom panel represents the scatterplot of normalized alpha power against SCP d.v. in single trials. Each color represents an individual subject (n = 11) and the bold black line represents the linear trend across subjects. (D) Timecourse of the mean SCP d.v. activity (n = 24) across the pre-stimulus interval for recognized (rec) and unrecognized (unrec) trials in the high-level data set. Shaded areas indicate the paired sem. (E) Topographic plots and timecourses for the 2 alpha power clusters obtained in the high-level data set. (F) Correlation between alpha power and SCP d.v. across single trials in the high-level data set. The top panel depicts the boxplots for z-transformed correlation coefficients across subjects for each of the 2 alpha power clusters. The bottom panel depicts the scatterplots for the correlation for the first alpha power cluster (light blue cluster) and the second alpha power cluster (dark blue cluster). Each color represents an individual subject (n = 24), and the bold black line represents the linear trend across subjects. The data underlying this figure can be found as Fig 2_Data at https://doi.org/10.5281/zenodo.14291607. d.v., decision variable; SCP, slow cortical potential.
Pre-stimulus alpha power predicts perceptual outcome
To assess the impact of alpha power on conscious perceptual outcome, we first extracted the oscillatory power in the alpha frequency range (7 to 14 Hz), separate from the aperiodic activity (i.e., the oscillatory power above and beyond the 1/f power spectrum [45–47]), in four 400-ms time windows within the pre-stimulus time interval (−1.7 to −0.1 s). We then compared pre-stimulus alpha power in each time window between perceived and unperceived trials at every sensor (Fig 1F).
In the low-level data set, we obtained a significant sensor cluster distributed over posterior occipital cortex, in which the pre-stimulus alpha power was significantly lower in seen than unseen trials in the time interval from −1.7 to −1.3 s (cluster-based permutation test; p Wcluster = −3,648; Fig 2B). The effect in this sensor cluster persisted across all pre-stimulus time windows but became weaker over time.
In the high-level data set, we obtained 2 significant sensor clusters, in the time interval of −1.3 to −0.9 s (p = 0.039, Wcluster = 8,092) and −0.5 to −0.1 s (p = 0.029, Wcluster = 9,256), respectively (Fig 2E). Both clusters were distributed anteriorly and had higher pre-stimulus alpha power in recognized than unrecognized trials—which was in the opposite direction of the pattern in the low-level data set.
The alpha power cluster identified in the low-level data set reflects a well-documented effect in the literature, whereby pre-stimulus alpha power in occipital regions negatively predicts conscious detection of low-level threshold stimuli—an effect interpreted as reflecting alpha power’s inverse relationship with cortical excitability in sensory regions [6,16,17,43]. Interestingly, the alpha power clusters in the high-level data set had an opposite effect and were located more anteriorly, suggesting that the relationship between alpha oscillatory power and conscious perception depends on the nature of the visual task. In the high-level data set, conscious recognition depends on the segmentation of a meaningful content, which may be less influenced by the excitability state of sensory regions [43]. Instead, we speculate that the effect seen in the high-level data set might reflect a positive relationship between alpha power and tonic alertness, which is regulated by the cingulo-opercular network located in more anterior regions [27,28], or the involvement of alpha oscillations in the top-down modulation of attention [48–50].
Pre-stimulus beta power predicts perceptual outcome
We then conducted a similar analysis to assess the impact of beta (14 to 30 Hz) power on conscious perceptual outcome. In the low-level data set, we obtained 2 pre-stimulus beta power clusters, both in the time interval from −1.7 to −1.3 s (cluster-based permutation test; cluster 1: p = 0.015, Wcluster = −1,678; cluster 2: p = 0.031, Wcluster = −1,330; Fig 3A). Both clusters were distributed over posterior regions and had lower pre-stimulus beta power in seen trials than unseen trials.
Fig 3. Beta power and SCP d.v. both influence perceptual outcome and do not covary in the low-level data set but do in the high-level data set.
(A) The topographic plots of the first (orange) and second (red) beta power clusters in the low-level data set are depicted on the left, along with the corresponding timecourses of beta power across the pre- and post-stimulus intervals on the right, for seen and unseen trials. The dotted vertical lines indicate the limits of the 4 temporal intervals considered during the analysis. The thick vertical red line indicates the time of stimulus onset. The shaded gray area indicates the pre-stimulus temporal interval during which beta power significantly differed between seen and unseen trials for the corresponding cluster. (B) Correlation between beta power and SCP d.v. across single trials in the low-level data set. The left panel depicts the scatterplots corresponding to each cluster. Each color represents an individual subject (n = 11), and the bold black line represents the linear trend across subjects. Boxplots on the right depict the z-transformed correlation coefficients across subjects for both clusters in the low-level data set, tested against 0 with a Wilcoxon signed-rank test; n.s.: not significant. Dashed horizontal lines represent a null correlation. (C) The topographic plots of the first (light green) and second (dark green) beta power clusters in the high-level data set are depicted on the left, along with the corresponding timecourses of beta power across the pre- and post-stimulus intervals on the right, for recognized and unrecognized trials. (D) Correlation between beta power and SCP d.v. across single trials in the high-level data set. The left panel depicts the scatterplots corresponding to each cluster. Each color represents an individual subject (n = 24), and the bold black line represents the linear trend across subjects. Boxplots depict the z-transformed correlation coefficients across subjects for each of the 2 beta power clusters in the high-level data set, tested against 0 with a Wilcoxon signed-rank test. Dashed horizontal lines represent a null correlation. For all boxplots, the solid line represents the median, and the edges indicate the quartiles; * p p ≤ 0.01. The data underlying this figure can be found as Fig 3_Data at https://doi.org/10.5281/zenodo.14291607. d.v., decision variable; SCP, slow cortical potential.
In the high-level data set, we also obtained 2 beta power clusters, in the time interval of −0.9 to −0.5 s (p = 0.027, Wcluster = 5,300) and −0.5 to −0.1 s (p Wcluster = 12,461), respectively (Fig 3C). Like for alpha power, both of these clusters were distributed more anteriorly and had higher pre-stimulus beta power in recognized than unrecognized trials.
Interestingly, the beta power clusters obtained in both data sets match the alpha power clusters in striking ways: in the low-level data set, both alpha and beta clusters had a more posterior location and had lower power preceding perceived trials; by contrast, in the high-level data set, both alpha and beta clusters had more anterior locations and had higher power preceding perceived trials. This suggests that the type of visual task modulates the specific impact of spontaneous brain oscillations in conscious perception.
Alpha power and SCP independently influence conscious visual perception
In the previous analyses, we first obtained a single-trial metric of perceptually relevant spontaneous aperiodic activity, in the form of SCP d.v., which measures the strength of evidence in the SCP range for or against conscious perception (with positive values predicting conscious perception). We then obtained single-trial metrics of perceptually relevant spontaneous oscillatory activity, in the form of power fluctuations in sensor clusters where pre-stimulus alpha or beta power predicts conscious perceptual outcome. Our study’s primary goal was then to assess whether aperiodic and oscillatory spontaneous activity have shared or independent influences on perception (Fig 1G).
To that end, we first assessed the relationship between perceptually relevant pre-stimulus SCP and alpha power activity. For each alpha power cluster (Fig 2B and 2E), we averaged pre-stimulus alpha power in the relevant time window across sensors within the cluster and correlated this cluster-wide power metric with the SCP d.v. across trials, in the same time window. We found that in the low-level data set, the correlation between alpha power and SCP d.v. was not significant (Wilcoxon signed-rank test across subjects; p = 0.15, W = −16; Fig 2C). A Bayesian one-sample t test yielded a Bayes Factor (BF10) of 0.828. This can be interpreted as weak evidence in favor of the null hypothesis.
Similarly, in the high-level data set, the correlation between alpha power and SCP d.v. was not significant in either cluster (p = 0.152, W = 99; p = 0.06, W = 83; Fig 2F). A Bayesian one-sample t test yielded a BF10 of 0.331 for cluster 1 (light blue in Fig 2E and 2F), which is considered weak-moderate evidence in favor of the null hypothesis, and a BF10 of 1.240 for cluster 2 (dark blue in Fig 2E and 2F), which is considered weak evidence in favor of the alternative hypothesis.
Together, these results suggest that although each spontaneous signal was individually linked to subsequent perceptual outcome, there was little evidence of a strong association between the signals, suggesting that their influences on perception are likely independent from each other.
Beta power and SCP independently influence conscious visual perception
Next, we assessed whether pre-stimulus beta power and SCP have shared influences on conscious perception. To this end, similar to the previous analysis, we first correlated SCP d.v. and beta power (averaged across sensors within a cluster) across trials for each subject, and assessed the significance of the correlation at the population level.
In the low-level data set, the correlation between beta power and SCP d.v. was not significant in either cluster (Wilcoxon signed-rank tests; p = 0.52, W = −25; p = 0.32, W = −21; Fig 3B). A Bayesian one-sample t test yielded a BF10 of 0.3 for both clusters, representing moderate evidence in favor of the null hypothesis. This result suggests that beta power and SCP, although with significant individual influences on perceptual outcome, are not significantly associated with each other, and hence likely operate independently on perception.
In the high-level data set, we found a significant positive correlation between beta power and SCP d.v. in both clusters (p = 0.037, W = 77; p = 0.009, W = 60; Fig 3D). A Bayesian one-sample t test yielded a BF10 of 1.835 for cluster 1 and a BF10 of 2.777 for cluster 2, which are considered weak evidence in favor of the alternative hypothesis. Given that beta power and SCP d.v. covaried in the high-level data set, we then investigated whether they had shared influences on perception. In principle, while shared perceptual influences require the covariation between 2 variables, it is important to note that such covariation does not necessarily indicate shared influences on perception.
To address this question, we constructed a linear regression model between beta power and SCP d.v., which allowed us to compute the residual of each signal when its shared variance with the other signal had been removed (Fig 4A, top). We then computed the ability of each signal, as well as its residual, to predict perceptual outcome using a receiver-operator curve (ROC) analysis (Fig 4A, bottom). The area under the ROC (AUROC) score from the ROC analysis quantifies the predictive power each signal has on perceptual outcome (see Methods). We then compared the AUROC scores between beta power and beta power residuals (once the shared variance with SCP d.v. had been removed) and between SCP d.v. and SCP d.v. residuals (once the shared variance with beta power had been removed).
Fig 4. AUROCs for beta power and SCP d.v. demonstrate that their influences on perceptual outcome are independent.
(A) Given that SCP d.v. and beta power were significantly positively correlated in the high-level data set, we modeled SCP d.v. and beta power as having shared variance, depicted by the overlap of the 2 circles in the top diagram. SCP d.v., including both independent variance and variance shared with beta power, is depicted in dark purple. SCP d.v. residuals, representing the variance in SCP d.v. once the shared variance with beta power has been removed, is depicted in light purple. Beta power, including both independent variance and variance shared with SCP d.v., is depicted in dark magenta. Beta power residuals, representing the variance in beta power once the shared variance with SCP d.v. has been removed, is depicted in light pink. The bottom panel represents a model ROC curve, in which the false positive rate (i.e., the rate at which a classifier will falsely label a trial as “recognized,” based on a given activity metric) is on the X-axis and the true positive rate (i.e., the rate at which a classifier will correctly label a trial as “unrecognized,” based on a given activity metric) is on the Y-axis. A random classifier will have an AUROC of 0.5, as depicted by the dashed gray line. Better classifiers will have AUROCs closer to 1. (B) AUROC scores for the first beta power cluster, comparing SCP d.v. (dark purple) and SCP d.v. residuals (light purple) when shared variance with beta power was removed (top panel) and comparing beta power (dark magenta) and beta power residuals (light pink) when shared variance with SCP d.v. was removed (bottom panel). (C) AUROC scores for the second beta power cluster, comparing SCP d.v. and SCP d.v. residuals (top panel) and beta power and beta power residuals (bottom panel). In B and C, dashed horizontal line represents the AUROC score of a random classifier. Scores were each compared to the random classifier value and to each other with Wilcoxon signed-rank tests. * p p ≤ 0.01, *** p ≤ 0.001, n.s.: not significant. The data underlying this figure can be found as Fig 4_Data at https://doi.org/10.5281/zenodo.14291607. AUROC, area under the ROC; d.v., decision variable; ROC, receiver-operator curve; SCP, slow cortical potential.
This analysis showed that, in the first beta cluster (Fig 4B), SCP d.v. and SCP d.v. residuals AUROC scores were not significantly different (Wilcoxon signed-rank test; p = 0.16, W = 92, BF10 = 0.875), and both SCP d.v. and SCP d.v. residual had AUROC scores significantly above the chance level of 0.5 (p W = 299, BF10 = 1.4e7; p W = 300, BF10 = 1.55e7, respectively). Similarly, the AUROCs for beta power and beta power residuals were not significantly different (p = 0.18, W = 102, BF10 = 0.536). The AUROCs for beta power and beta power residual had trend-level significance when compared to 0.5 (p = 0.0501, W = 208, BF10 = 2.021; p = 0.0604, W = 205, BF10 = 1.35, respectively), which was likely due to the process of averaging beta power across sensors within the cluster, diluting the predictive power of beta power for perceptual outcome that was initially measured at the individual sensor level. Critical to our question, the AUROC scores did not differ between each signal and its residual, suggesting that removing the shared variance between beta power and SCP d.v. did not degrade either signal’s predictive power for perception.
In the second beta cluster (Fig 4C), the AUROC scores for SCP d.v. and SCP d.v. residuals were not significantly different (p = 0.1, W = 92, BF10 = 0.665), and both were significantly higher than the chance level (p W = 291, BF10 = 2e5; p W = 291, BF10 = 1.6e5, respectively). Similarly, the AUROCs for beta power and beta power residuals were not significantly different (p = 0.47, W = 124, BF10 = 0.308), and both scores were significantly above the chance level (p W = 257, BF10 = 66.6; p BF10 = 1423, respectively).
Together, these results show that, for both beta power and SCP d.v., when the variance they share with each other is removed, their ability to predict perceptual outcome is unchanged. This demonstrates that pre-stimulus beta power and SCP d.v. explain non-overlapping variance in the perceptual outcome, suggesting that they independently contribute to conscious perception.
Alpha power and beta power have shared influences on perception
We next investigated whether alpha and beta power have shared influences on perception. To this end, we first investigated across-trial correlation between alpha power and beta power, using all pairs of significant clusters identified in the earlier analyses (Fig 5, top panels). Thus, we tested whether the pre-stimulus alpha power that predicted perception and the pre-stimulus beta power that predicted perception co-fluctuated from trial to trial. We found that alpha and beta power were positively correlated in all cluster pairs of both data sets (Wilcoxon signed-rank test; all p Fig 5, bottom panels).
Fig 5. Alpha power and beta power are positively correlated.
(A) Correlation between alpha power and beta power across single trials, between alpha cluster 1 and beta cluster 1 (left bar) and between alpha cluster 1 and beta cluster 1 (right bar) of the low-level data set. (B) Correlation between alpha power and beta power across single trials, between all pairs of alpha-beta clusters of the high-level dataset. In both A and B, boxplots represent z-transformed correlation coefficients across subjects and were tested against 0 with a Wilcoxon signed-rank test. For all boxplots, the solid line represents the median and the edges indicate the quartiles; * p p ≤ 0.01, *** p ≤ 0.001. The data underlying this figure can be found as Fig 5_Data at https://doi.org/10.5281/zenodo.14291607.
To address whether alpha and beta power had shared influences on perceptual outcome, similar to the previous analysis between beta power and SCP, we computed the alpha power AUROC scores and compared them with the AUROC scores for alpha power residuals once its shared variance with beta power had been removed. Similarly, we compared AUROC scores for beta power and beta power residuals.
In the low-level data set (Fig 5A), we found that the scores for alpha power and alpha power residuals after removing variance from both beta clusters did not significantly differ (Wilcoxon signed-rank test; p = 0.28, W = 1.08). The AUROC scores were significantly higher than the chance level of 0.5 for alpha power (p = 0.0034) but not for its residuals (p = 0.087), suggesting that some of the variance in perceptual outcome explained by alpha power is lost when beta power is regressed out, even though the differences were not statistically significant. Further, for both beta clusters, we found that the AUROC scores for beta power and beta power residuals after removing shared variance with the alpha cluster had marginally significant differences (beta cluster 1: p = 0.071, W = 1.81; beta cluster 2: p = 0.053, W = 1.94). The AUROC scores were not significant for beta power residuals (beta cluster 1: p = 0.83; beta cluster 2: p = 0.92, W = 1.94). These results suggest that in the low-level data set, alpha and beta power had significantly shared variance in their influences on perception.
In the high-level data set (Fig 5B), scores for alpha power and alpha power residuals after removing variance from both beta clusters did not significantly differ in alpha cluster 1 (p = 0.21, W = 1.26) but did in alpha cluster 2 (p = 0.027, W = 2.21). The AUROC scores were significantly higher than a chance level of 0.5 for alpha power and alpha power residuals (p p = 0.35, W = 0.93; beta cluster 2: p = 0.38, W = 0.89). The AUROC scores were all significantly higher than a chance level of 0.5 (p
Together, these results suggest that, unlike the relationship between oscillatory power and SCP, alpha power and beta power did have partially shared influences on perception.
Oscillatory power and SCPs correlate with pupil size
Arousal fluctuates constantly within the normal wakefulness state and can be tracked by the moment-to-moment changes in pupil diameter [39,51]. Spontaneous fluctuations of pupil size track the firing rates of locus coeruleus (LC) neurons, part of the ascending arousal system, which send widespread noradrenergic projections to the cortex [52]. Previous studies have shown that pre-stimulus baseline pupil size influences perceptual decision-making, including both reaction times and discrimination accuracy [30,53]. Using the high-level data set investigated herein, we previously reported that pupil size in a 2-s pre-stimulus window predicts subjective recognition of object images from trial to trial and modulates widespread cortical activity power measured by MEG [37]. These observations motivated us to hypothesize that the present findings, showing independent influences of oscillations and SCPs on perception, could be better understood by considering their respective relationships with pupil-linked arousal.
To investigate this question, we utilized the high-level data set, which had concurrent pupillometry and MEG recordings. For each pre-stimulus neural activity found to influence perception, we tested whether it was significantly correlated with pre-stimulus pupil size from trial to trial. Using the alpha clusters identified in the earlier analysis (Fig 2E), we found that alpha power from both sensor clusters were positively correlated with pupil size (Wilcoxon signed-rank test: p = 0.023, W = 64, BF10 = 4.114; p = 0.012, W = 57, BF10 = 5.416, respectively; Fig 6A). Similarly, we found that beta power in both sensory clusters (Fig 3C) positively correlated with pupil size (p = 0.0014, W = 38, BF10 = 25.189; p W = 28, BF10 = 171.046, respectively; Fig 6B). Finally, the SCP d.v. was also significantly correlated with pupil size from trial to trial (p W = 30, BF10 = 63.066; Fig 6C).
Fig 6. Alpha and beta oscillations, as well as SCP d.v., positively correlate with pupil size.
(A) Correlation between alpha power and pupil size across single trials, for both alpha power clusters of the high-level data set. (B) Correlation between beta power and pupil size across single trials, for both beta power clusters of the high-level data set. (C) Correlation between SCP d.v. and pupil size across the entire pre-stimulus interval in the high-level data set. In A–C, boxplots represent z-transformed correlation coefficients across subjects and were tested against 0 with a Wilcoxon signed-rank test. Dashed horizontal lines represent a null correlation. The solid line represents the median and the edges indicate the quartiles. * p p ≤ 0.01, *** p ≤ 0.001. The data underlying this figure can be found as Fig 6_Data at https://doi.org/10.5281/zenodo.14291607. d.v., decision variable; SCP, slow cortical potential.
Since both alpha and beta power, as well as SCP d.v., were higher in the pre-stimulus period in recognized than unrecognized trials in the high-level data set (Figs 2D, 2E and 3C), their positive correlations with pupil size were consistent with an overall positive relationship between pupil size and recognition rate [37]. These results raise the question of the specific relationship between neural activity and pupil size’s influences on perception, which we probe in detail below.
Oscillatory power and SCP’s influences on perception have distinct relationships to pupil size
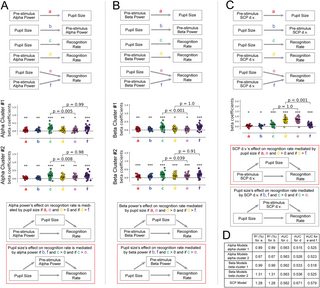
Given that all 3 neurophysiological signals were correlated with pupil size, we next investigated the relationship between their perceptual influences and the influence of pupil-linked arousal on perception. Specifically, are oscillatory power/SCP’s influences on conscious perception mediated by pupil-linked arousal, or, alternatively, are they the mediators of pupil-linked arousal’s influences on perception? To answer this question, we conducted 3 sets of mediation analyses (for details, see Methods) to test the respective relationship of alpha power, beta power, and SCP d.v. with pupil size in their perceptual influences.
The first set of mediation analyses concerned the relationship between pre-stimulus alpha power, pre-stimulus pupil size, and recognition rate (Fig 7A). We first tested whether pre-stimulus alpha power’s influence on the recognition rate was mediated by pupil size, in each of the alpha clusters (shown in Fig 2E). To do so, we first tested the basic conditions of mediation: we assessed the direct effect between pre-stimulus alpha power and pupil size (a), the direct effect between pre-stimulus alpha power and recognition rate (d), and the indirect effect between pupil size and recognition rate, when pre-stimulus alpha power was controlled for (e). In the first alpha cluster, we found that coefficients a (one-sample t test: t22 = 3.30, p = 0.0033), d (t22 = 2.98, p = 0.007), and e (t22 = 2.30, p = 0.031) were all significantly positive. Since these conditions were met, to find evidence of mediation, the indirect effect between pre-stimulus alpha power and recognition rate, when controlling for pupil size (f), must be smaller than the direct effect between pre-stimulus alpha power and recognition rate (d). We thus assessed whether d > f and found that the difference was not significant (t22 = −2.69, p = 0.99, BF10 = 0.071). We tested the same model in the second alpha cluster and found that all 3 basic conditions were met (t22 = 3.27, p = 0.003; t22 = 3.06, p = 0.006; t22 = 2.91, p = 0.008 for a, d, and e, respectively), but again the mediation effect was not significant (t22 = −2.3, p = 0.98, BF10 = 0.078). Therefore, we found no evidence of mediation between pre-stimulus alpha power and recognition rate via pupil size, in either alpha cluster (model indicated by the gray box in Fig 7A).
Fig 7. Alpha power, beta power, and SCP d.v. are related to pupil size, which is thought to reflect arousal.
(A) The first set of mediation analyses explored the relationship between pre-stimulus alpha power, pupil size, and recognition rate. Model coefficients for the direct and indirect effects depicted in the top panel were computed and are plotted below for each alpha cluster. All initial conditions (see bottom panel) for both mediation models were met, but only the mediation effect for the model in which pupil size influences recognition rate via pre-stimulus alpha power was significant, as depicted by the red box. (B) The second set of mediation analyses explored the relationship between pre-stimulus beta power, pupil size, and recognition rate. Model coefficients for the direct and indirect effects depicted in the top panel were computed and are plotted below for each beta cluster. All initial conditions (see bottom panel) for both mediation models were met, but only the mediation effect for the model in which pupil size influences recognition rate via pre-stimulus beta power was significant, as depicted by the red box. (C) The third set of mediation analyses explored the relationship between pre-stimulus SCP d.v., pupil size, and recognition rate. Model coefficients for the direct and indirect effects depicted in the top panel were computed and are represented below. All initial conditions (see bottom panel) for both mediation models were met, but only the mediation effect for the model in which SCP d.v. influences recognition rate via pupil size was significant, as depicted by the red box. For all mediation analyses in A–C, direct effects between the 3 variables were captured with linear regression coefficients for a and b and logistic regression coefficients for c and d. Indirect effects of both predictor variables and recognition rate were captured with multiple logistic regression coefficients e and f. All coefficients are standardized. Boxplots represent z-transformed correlation coefficients across subjects and were tested against 0 with a Wilcoxon signed-rank test. Dashed horizontal lines represent a null correlation. For all boxplots, the solid line represents the median and the edges indicate the quartiles. * p p ≤ 0.01, *** p ≤ 0.001. (D) Table containing metrics for assessing model fits for each of the direct and indirect effects computed for all mediation analyses. Model fits for all linear regression models are assessed with the R2 value in percentage points. Model fits for all logistic regression models are assessed with the AUC. The data underlying this figure can be found as Fig 7_Data at https://doi.org/10.5281/zenodo.14291607. AUC, area under the curve; d.v., decision variable; SCP, slow cortical potential.
We then tested the alternative mediation model, whereby pupil size’s influence on recognition rate is mediated by pre-stimulus alpha power. To do so, we first tested the basic conditions of mediation: we checked that the direct effect between pupil size and pre-stimulus alpha power (b), and between pupil size and recognition rate (c) and the indirect effect of pre-stimulus alpha power on recognition rate, controlling for pupil size (f) were significant. We found that all 3 conditions were met in the first alpha cluster (t22 = 3.30, p = 0.003; t22 = 4.76, p t22 = 4.75, p b, c, and f, respectively) and in the second alpha cluster (t22 = 3.27, p = 0.003; t22 = 4.86, p t22 = 4.77, p b, c, and f, respectively). We then tested the mediation effect by assessing whether the indirect effect between pupil size and recognition rate, when controlling for pre-stimulus alpha power (e), was smaller than the direct effect between pupil size and recognition rate (c). We found significant evidence for mediation (i.e., c > e) in both alpha clusters (t22 = 2.78, p = 0.005, BF10 = 7.29 for alpha cluster 1, t22 = 2.63, p = 0.008, BF10 = 5.58 for alpha cluster 2). Therefore, the influence of pupil size on recognition rate is mediated by pre-stimulus alpha power, in both alpha clusters (red box, Fig 7A).
The second set of mediation analyses concerned the relationship between pre-stimulus beta power, pupil size, and recognition rate (Fig 7B). We first tested whether pre-stimulus beta power’s influence on the recognition rate was mediated by pupil size, in each of the beta clusters (shown in Fig 3C). We found that the 3 basic conditions for mediation were met in the first cluster (t22 = 3.58, p = 0.002; t22 = 2.74, p = 0.012; t22 = 2.52, p = 0.02 for a, d, and e, respectively) and in the second cluster (t22 = 4.60, p t22 = 4.40, p t22 = 3.99, p a, d, and e, respectively). We then tested for the possibility that beta power’s influence on perception was mediated by pupil size (i.e., d > f) and found no evidence of mediation in either cluster (t22 = −3.41, p = 1.0, BF10 = 0.062; t22 = −1.42, p = 0.91, BF10 = 0.103).
We then tested the alternative mediation model, whereby pupil size’s influence on recognition rate is mediated by pre-stimulus beta power. We also found the 3 basic conditions for mediation were met in the first cluster (t22 = 3.58, p = 0.002; t22 = 4.86, p t22 = 4.88, p b, c, and f, respectively) and in the second cluster (t22 = 4.60, p t22 = 4.86, p t22 = 4.75, p b, c, and f, respectively). We then tested for the possibility that pupil’s influence on perception was mediated by beta power (i.e., c > e) and found significant evidence of mediation in both clusters (t22 = 3.72, p BF10 = 43.38 for beta cluster 1, t22 = 1.85, p = 0.039, BF10 = 1.60 for beta cluster 2). Therefore, the influence of pupil size on recognition rate is also mediated by pre-stimulus beta power, in both beta clusters (Fig 7B, red box).
The third set of mediation analyses concerned the relationship between pre-stimulus SCP d.v., pupil size, and recognition rate (Fig 7C). We first tested whether the influence of pre-stimulus SCP d.v. on recognition rate was mediated by pupil size. We found that the 3 basic conditions for mediation were met (t22 = 2.72, p = 0.013; t22 = 11.01, p t22 = 10.93, p a, d, and e, respectively). We tested the mediation effect (i.e., d > f) and found significant evidence of mediation (t22 = 3.72, p BF10 = 2.08e5). These results suggest that the influence of SCP d.v. on recognition rate is mediated by pupil size (Fig 7C, red box).
We then tested the alternative mediation model, whereby pupil size’s influence on recognition rate is mediated by pre-stimulus SCP d.v. We also found that the 3 basic conditions for mediation were met (t22 = 2.72, p = 0.013; t22 = 4.88, p t22 = 4.27, p b, c, and f, respectively). We tested the mediation effect (i.e., c > e) and found no evidence of mediation (t22 = −7.41, p = 1.0, BF10 = 0.017).
In all analyses, the overall performance of each of the models was quantified using the coefficient of determination R2 for linear regression models and using AUC (area under the ROC curve) for logistic regression models, as summarized in Fig 7D. Together, these findings suggest that oscillatory powers and SCPs have distinct relations to pupil-linked arousal in their influences on conscious perception. While SCP d.v.’s influence on conscious perception is mediated by pupil-linked arousal, pupil-linked arousal’s perceptual influence is itself mediated by the wax-and-wane of alpha and beta oscillations (Fig 7, red outlines).
Discussion
Spontaneous power fluctuations of brain oscillations and spontaneous variations in large-scale SCP activity patterns have both been found to predict conscious perception. Here we show, for the first time, that their influences on perception act through independent channels. In addition, while SCP’s perceptual influence was partially mediated by pupil-linked arousal, alpha and beta powers acted as partial mediators of pupil-linked arousal’s influence on perception. These results establish that aperiodic brain activity and brain oscillations have independent influences on perception. Importantly, our main findings showing independence between pre-stimulus SCP and oscillation’s effects on perception were reproduced in 2 separate data sets involving different visual stimuli, showing the reproducibility and generalizability of the present findings. In what follows, we discuss the implications of these findings.
In both data sets, conscious perceptual outcome, whether it was the detection of a Gabor patch or the recognition of a meaningful content, could be decoded from the whole-brain pattern of SCPs in the pre-stimulus interval [10,12]. SCPs correspond to fluctuations in brain field potentials comprising the low-frequency end (44,54]. Indeed, tasks presented on the negative shifts of spontaneous SCP fluctuations are solved faster [55,56], more accurately [57], and have a lower sensory threshold [34]. Further, negative SCPs in the pre-stimulus interval were shown to be associated with the awareness level of a visual stimulus [58]. Although the neural mechanism by which spontaneous SCPs modulates perceptual awareness is not yet fully understood, our previous study using the high-level data set showed that the SCP d.v. extracted using the present approach predicts conscious recognition in a “non-content-specific” manner [12], such that the same pre-stimulus activity pattern facilitates recognition of images from different categories. This observation is compatible with the present finding that SCP’s influence on conscious perception is at least partially mediated by pupil-linked arousal mechanisms (Fig 7C). We note that this earlier study [12] also observed a “content-specific” process in the pre-stimulus SCPs, where a different activity pattern promotes the recognition of each object category, and this content-specific SCP activity was not correlated with pupil size. Our study did not probe the content-specific spontaneous SCP process, because whether there are similar processes in spontaneous alpha and beta power fluctuations that influence perception in a content-specific manner remains to be seen.
Concerning alpha oscillations, the most frequently documented effect on conscious perception is that pre-stimulus alpha power in occipital regions is inversely related to the probability of consciously perceiving a visual stimulus [6,13–17]. Here, in the low-level data set, we replicated this effect: alpha power in a group of occipital sensors was significantly lower preceding consciously detected stimuli (Fig 2B). In line with previous findings, this likely reflects excitability fluctuations in visual areas that influence how an incoming stimulus is processed. Supporting this interpretation, occipital alpha power predicts the probability of perceiving a phosphene elicited by a TMS pulse, a phenomenon known to vary as a function of visual cortex excitability [59,60]. Changes in excitability gated by alpha oscillations could reflect waves of inhibition in sensory regions [61,62], such that lower alpha power indicates a disinhibition that facilitates sensory processing.
In the high-level data set, we observed a different pattern, with higher alpha power preceding consciously recognized stimuli, accompanied by a distinct spatial topography centered in more anterior regions. This suggests a distinct mechanism through which alpha oscillations may influence visual perception. In the high-level data set, instead of detecting a visual signal from a uniform background where the excitability of sensory regions might play a stronger role (as in the low-level data set), subjects’ task was to detect a meaningful content in a complex visual image which requires object segmentation. Here, top-down gating or feedback from higher-order areas likely play a more important role. Alpha oscillations have been implicated in attention- and prediction-mediated facilitation of specific visual representations [19,48–50,63–65]. For instance, pre-stimulus feedback connectivity in the alpha range can bias the content of visual perception [66]. Additionally, shifts in attention can modulate parietal excitability via changes in alpha power [48]. Further, pre-stimulus alpha power in frontoparietal areas predicts individual perceptual biases [67,68]. These studies collectively support a role for frontoparietal alpha power in mediating top-down signals that regulate perception and are consistent with models suggesting a role for frontal alpha power in cognitive control [27].
Another, not mutually exclusive possibility is that the perceptually relevant alpha power we identified in the high-level data set might originate from the cingulo-opercular network that controls tonic alertness [27,28]. This interpretation is consistent with our finding that alpha power partially mediates pupil-linked arousal’s influence on recognition rates. Importantly, spontaneous activation of the anterior insular cortex, a key node of the cingulo-opercular network, precedes spontaneous phasic pupil dilation [69], and these brain regions have been suggested to be part of the “central autonomic network” involved in controlling the autonomic nervous system which in turn controls spontaneous changes in pupil size [69].
Importantly, despite the notable differences in the specific relations between alpha oscillations and conscious perception in the 2 data sets, in both cases we found that alpha power and SCP d.v. were not correlated with each other, even though both signals were predictive of perceptual outcome. This suggests that these 2 measures of spontaneous activity likely have independent sources and mechanisms of action on perception. This conclusion is consistent with models suggesting that 1/f aperiodic activity and oscillations add on top of each other in both the time domain and frequency domain (the “additive model”) and have separate sources [45,70].
Similarly, for beta oscillations, we found that beta power and conscious perception were inversely related in the low-level data set, and positively related in the high-level data set—again, with more anteriorly located clusters in the high-level data set. The underlying mechanisms of beta power’s influences on visual perception are far less studied than for alpha oscillations. Beta oscillations have been implicated in perceptual expectations [71] and the reactivation of content-specific perceptual representations [72,73]. Additionally, an earlier study reported a causal role for beta power in the frontoparietal network in conscious visual perception [74]. Importantly, here too, despite different effects of beta power on conscious perception as a function of the visual task, we found no significant relationship between perceptually relevant measures of beta power and SCPs. Although we did find a positive correlation between beta power and SCP d.v. in the high-level data set, our follow-up analysis showed that each measure accounted for non-overlapping variance in the perceptual outcome, suggesting that they exerted independent influences on perception.
Finally, in the high-level data set which had concurrent MEG and pupil recordings, we found that pre-stimulus alpha power, beta power, and SCP d.v. all correlated with pre-stimulus pupil size across trials. This finding indicates that all 3 measures of spontaneous brain activity may be related to fast changes in arousal within the waking state [39,51], despite the mutual independence of their perceptual influences. We probed this issue further using a series of mediation analyses, which revealed distinct relationships of oscillatory power and SCP with regard to pupil size in their influences on perception.
We found that pre-stimulus alpha and beta power partially mediate pre-stimulus pupil size’s influence on recognition (Fig 7A and 7B). For alpha power, this effect can be potentially explained by its relationship to tonic alertness, as discussed earlier. In addition, a recent study showed that noradrenergic activity—which is a central mechanism in controlling pupil size—is associated with changes in alpha power [75]. Beta power’s effect could potentially be explained by its strong correlation with alpha power’s influence on perception (Fig 5). Our observation of a positive correlation between alpha power and pupil size is consistent with a recent study [76]. This study, using a range of stimulus contrasts, revealed that alpha power and pupil size had, respectively, an additive versus a multiplicative influence on detection across stimulus contrast levels [76]. Our finding showing that alpha power partially mediated pupil’s influence on perception is not incompatible with this earlier study and might help to explain earlier findings showing that baseline pupil size can influence both the sensitivity and criterion of conscious detection [37].
We also found that SCP’s influence on conscious recognition was at least partly mediated by pupil size. This suggests that the large-scale SCP activity patterns modulate the pupil-linked arousal system, which may in turn influence conscious perception. SCPs may influence the activation of the locus coeruleus-norepinephrine (LC-NE) system through top-down cortical projections, thereby influencing pupil size. In turn, shifts in pupil-linked arousal can influence visual perception through a variety of neural mechanisms, including the release of NE and acetylcholine in the cortex [52,77,78], which in the visual cortex could enhance the signal-to-noise ratio during visual processing [38]. In addition, pupil-linked arousal changes could enhance thalamic function [79,80] such that visual stimuli are more readily processed. Further research is needed to establish the precise neural mechanisms governing the complex interplay between brain oscillations, SCPs, physiological arousal, and visual awareness.
In summary, our results, obtained using 2 data sets involving different visual tasks and stimuli, demonstrate that spontaneous aperiodic fluctuations and oscillatory activity influence conscious perception through independent mechanisms. They also highlight the complex relationships between spontaneous neural activity and arousal in modulating conscious perception. These results shed new light on our understanding of how visual awareness arises through intricate interactions between spontaneous ongoing brain activity and incoming sensory inputs and pave the way for future dissection of these mechanisms through causal manipulations or circuit-level studies.
ADVERTISEMENT:
Hai, para pengemar slots Pernah denger istilah “slot demo”? Kalau belum, siap-siap jatuh cinta sama konsep ini. raja slot merupakan mesin slot yang sering memberi win. Ya, mesin-mesin ini bisa dibilang sebagai andalannya buat bawa come back cuan. but, cemana sih
tekniknya nemuin slot gaco yang tepat? Tenang Bro, kita bahas santai aja di tempat ini
Gaming terbaik waktu ini hanya satu berada Indonesia hanya di pasti memberikan imbal hasil terbaik
SEGERA hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia