Abstract
The major pathological feature of Parkinson ‘s disease (PD), the second most common neurodegenerative disease and most common movement disorder, is the predominant degeneration of dopaminergic neurons in the substantia nigra, a part of the midbrain. Despite decades of research, the molecular mechanisms of the origin of the disease remain unknown. While the disease was initially viewed as a purely neuronal disorder, results from single-cell transcriptomics have suggested that oligodendrocytes may play an important role in the early stages of Parkinson’s. Although these findings are of high relevance, particularly to the search for effective disease-modifying therapies, the actual functional role of oligodendrocytes in Parkinson’s disease remains highly speculative and requires a concerted scientific effort to be better understood. This Unsolved Mystery discusses the limited understanding of oligodendrocytes in PD, highlighting unresolved questions regarding functional changes in oligodendroglia, the role of myelin in nigral dopaminergic neurons, the impact of the toxic environment, and the aggregation of alpha-synuclein within oligodendrocytes.
Citation: Salazar Campos JM, Burbulla LF, Jäkel S (2025) Are oligodendrocytes bystanders or drivers of Parkinson’s disease pathology? PLoS Biol 23(1):
e3002977.
https://doi.org/10.1371/journal.pbio.3002977
Published: January 8, 2025
Copyright: © 2025 Salazar Campos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: L.F.B. is supported by an “Impact Award” from the Parkinson’s Foundation (PF-IMP-935999) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations::
GWAS,
genome-wide association studies; iPSC,
induced pluripotent stem cell; MRI,
magnetic resonance imaging; OPC,
oligodendrocyte progenitor cell; PD,
Parkinson’s disease; SNP,
single-nucleotide polymorphism
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with a largely unknown etiology, with around 90% to 95% of cases being of sporadic origin. Estimated to affect 1% of the population above age 65 and 4% of the population above 85, it is the second most prevalent neurodegenerative disease and the most common movement disorder [1]. The two primary pathological hallmarks are the selective degeneration of dopaminergic neurons in the substantia nigra, and the accumulation and aggregation of the protein alpha-synuclein (α-synuclein) in Lewy bodies and Lewy neurites within neurons (Fig 1A and 1B) [1]. These features are integral to certain neurodegenerative processes, particularly in PD and Lewy body dementia. α-Synuclein is a small, soluble protein primarily found in the presynaptic terminals of neurons, where it is involved in regulating neurotransmitter release. In neurodegenerative diseases, this protein misfolds and aggregates, losing its normal function and becoming toxic to cells [2].
Fig 1. Disease modeling in PD.
PD is a complex neurodegenerative human pathology that on the tissue level (A) is characterized by the loss of dopaminergic neurons in the substantia nigra in the human midbrain. On the cellular level (B), the major hallmark of PD pathology is the intracellular accumulation of α-synuclein into Lewy bodies and Lewy neurites in dopaminergic neurons and dopaminergic neuron degeneration. To model PD pathology, many genetic and toxin-induced mouse models (reviewed in [5]) have been developed (C). However, neither of those fully recapitulates human pathology, which is also likely due to species-specific differences between rodent and human biology [6]. Only a few models have specifically examined oligodendrocytes [7]. Therefore, human iPSC-derived 2D [8] and 3D models have been developed, including models with oligodendrocytes [9] with increasingly complex cellular compositions (D). Created with BioRender.com. iPSC, induced pluripotent stem cell; PD, Parkinson’s disease.
The neurodegeneration associated with α-synuclein, Lewy bodies, and Lewy neurites involves multiple mechanisms, i.e., pathological α-synuclein interfering with proper protein folding, mitochondrial function, neurotransmission, inflammation, and axonal transport of molecules and organelles. These processes collectively enhance neuronal vulnerability, particularly in regions like the substantia nigra, which results in the typical motor symptoms seen in PD [2]. Despite numerous advances in technologies and platforms, such as single nucleus-RNA sequencing [3], CRISPR gene editing, and induced pluripotent stem cell (iPSC)-derived disease modeling [4], the etiology of the disease remains elusive.
Initially, PD was viewed as a purely neuronal disease, which until recent years, directed research predominantly along a neurocentric path. However, with the rise of next-generation sequencing technologies, oligodendrocytes are increasingly coming into focus. Oligodendrocytes are highly abundant glial cells in the central nervous system, responsible for myelinating axons to facilitate fast saltatory nerve conduction and for providing support essential for neuronal function and long-term survival [10]. Oligodendrocytes have recently been suggested to play a significant role in the early stages of PD, as revealed by a strong link to PD risk genes and early transcriptional changes [11], which is further supported by evidence of structural changes in the white matter of patient brains [12,13]. As a significantly large volume of the white matter is comprised of myelin, these structural changes are believed to result, at least to a significant extent, from pathological alterations to oligodendrocytes. The functional relevance of these transcriptomic changes and their mechanistic effects are probably the most important open questions in the field at present.
Dopaminergic neurons in the substantia nigra have always been considered to be poorly myelinated [14]. Given the technological advances and the high abundance of oligodendrocytes in this area [15], this long-standing assumption may need to be re-evaluated, especially in the context of PD pathogenesis. Studying the non-myelinating functions of oligodendrocytes and their progenitor cells may also be of utmost importance in the future.
In this Unsolved Mystery, we provide a summary of the current, albeit limited, knowledge on oligodendrocytes in the pathogenesis of PD, discussing why they have remained such an enigma, and outline the necessary steps for future research to fully understand their functional implications. A better understanding of the non-cell-autonomous determinants of neuron vulnerability will be crucial for the development of novel approaches to disease-modifying therapies.
Why has the role of oligodendrocytes in PD pathogenesis remained so elusive?
While postmortem brain tissue has revealed many insights into pathological aspects of PD, this approach is limited to a single snapshot of the late stage of the disease. The identification of pathological mechanisms heavily relies on suitable animal models in which the progress of disease can be studied in various experimental setups and in a time-dependent manner.
Rodent models for studying neurodegenerative diseases have become well established over the past 20 years, particularly genetic and toxin-induced models that recapitulate various aspects of the disease (reviewed in [5]) (Fig 1C). These models have indeed provided invaluable insights into the pathogenesis of PD, however with a strong focus on neurons. Animal models focusing on oligodendrocytes and their potential contribution to neuronal death and other pathological hallmarks of the disease are sparse. In a synthetic α-synuclein fibril-induced mouse model, one study reported the involvement of oligodendrocytes in α-synuclein processing and pathology progression in vivo, indicating that indeed oligodendrocytes may contribute to disease progression [7]. However, the inability of these models to fully recapitulate pathological hallmarks of the disease—especially in regard to oligodendrocytes—remains a major obstacle to the discovery of translational therapies.
Alternatively, in vitro models using iPSCs have been advanced to uncover the pathogenic mechanisms of PD (see Fig 1D and [4,8,16]), with a primary focus on neurons. However, ongoing advancements in these technologies offer exciting opportunities to explore both cell-autonomous and non-cell-autonomous effects of oligodendrocytes in PD (see Box 1).
Box 1: Advantages and disadvantages of using stem cell-derived models for PD research
Human iPSCs have gained increasing attention in scientific research since their discovery as this approach opened up an entirely new field of research for human disease modeling without the ethical concerns associated with the use of human embryonic stem cells. Not only does it allow modeling different aspects of the disease, including the generation of different cell types, but it has also paved the way for the development of more personalized medicine approaches, as data can theoretically be obtained from each individual patient.
Still, there are many critical views that iPSC-derived models retain a stem cell character even during differentiation and scarcely show signs of aging, which is a key feature of most neurodegenerative diseases, including PD. Unlike iPSC-derived neurons, oligodendrocytes can currently only be cultured in vitro for a very limited time before they begin to die, potentially making the problem of the lack of an aging signature in these cells even more pronounced.
Nevertheless, techniques such as direct cellular reprogramming offer an alternative way for producing lineage-specific terminal cells by transforming fully differentiated adult cells directly into a specific cell type, bypassing the pluripotent stage. Unlike the iPSC reprogramming technique, which resets the cell’s epigenetic information by reverting them to pluripotency, direct conversion focuses on introducing the epigenetic characteristics of the target cell. This allows the preservation of aging signatures [17,18] and their further development will greatly benefit the field of neurodegeneration. However, protocols to directly reprogram somatic cells into oligodendrocytes are yet to be established.
Protocols for the generation of iPSC-derived oligodendrocytes are still relatively rare in comparison to that of other cell types and difficult to establish, especially in co-culture systems with other cells. During development of the human brain, oligodendrocytes develop quite late and myelination only starts after birth, when most other cell types and structures have already been established [19]. Being made relatively late in the process of development is likely an explanation for why original protocols for generating iPSC-derived oligodendrocytes are lengthy [20,21]. Other, and significantly faster protocols that utilize overexpression of key oligodendroglial transcription factors [22,23] have already proven efficient, including in the context of patient-derived cell lines [9]. However, the generation of these genetically modified, or lentiviral-induced cell lines is laborious and difficult to reproduce due to variable virus integration site and transduction efficiency. Therefore, these protocols cannot be easily applied to different patient-derived cell lines that have not been modified. Oligodendrocytes are even more difficult to obtain in more complex 3D platforms, such as spheroids and organoids (Fig 1D). High numbers of oligodendrocytes are usually absent in these models, and although a few studies have shown active myelination in their model system [24,25], this key physiological property is generally lacking. Thus, generating more robust models that include physiological numbers of myelinating oligodendrocytes to fully represent their function in the human brain should be of highest priority in the future for oligodendrocyte-related research.
Oligodendrocytes in Parkinson’s disease pathology: What do we know?
Magnetic resonance imaging (MRI) studies have shown that PD patients with dementia have significant structural changes in the majority of white matter tracts of the brain [12,13], often interpreted as demyelination. Although this type of measurement cannot directly assess the myelin content in patients, it may indicate cellular oligodendroglial changes or even a decline in the number of oligodendrocytes. Examining the peripheral nervous system, one study observed a significant loss of unmyelinated axons in the epicardial nerve of PD patients [26]. Even though this study does not strictly involve oligodendrocytes, as they only reside in the central nervous system, it highlights the neuroprotective function of myelin in the peripheral nervous system. In addition to the main pathological hallmarks, brain-wide oxidative stress is also an important feature of PD, and oligodendrocytes and oligodendrocyte progenitors cells (OPCs) are known to be highly vulnerable to oxidative damage [27]. This is due to a combination of high metabolic rates with potentially dangerous by-products, high intracellular iron, and low concentrations of antioxidants [28], further indicating that oligodendrocyte death may occur and contribute to the exacerbation of neuronal pathology.
Perhaps surprising for the field was the discovery of α-synuclein inclusions in oligodendrocytes in samples from PD patient brains [29,30]. This protein is thought to be expressed primarily in neurons, where it is known to regulate synaptic functions, and was therefore not expected to be present in oligodendrocytes. The source of α-synuclein remains under debate, as single nucleus RNA-sequencing approaches revealed endogenous expression of SNCA, the gene that encodes for α-synuclein, in oligodendrocytes in the human brain [31]. Oligodendrocytes from a rodent immortalized cell line are also capable of taking up monomeric and oligomeric forms of this protein in vitro [32]. Although this phenomenon is not very well defined in the context of PD, oligodendroglial α-synuclein inclusions are the main hallmark of multiple system atrophy, a rare neurodegenerative disease which also presents with parkinsonism [33]. Given that these diseases share some clinical and histopathological features, the question naturally arises as to how oligodendrocytes may be affected in PD and how they, in turn, respond to the pathological progression of the disease.
Imaging- and histology-based techniques for assessing oligodendrocytes in the context of PD are sparse, and interpretations of how these observations relate to the pathological developments are difficult. Due to technological advances in next-generation sequencing, studying oligodendroglial transcriptomic signatures in human patients has become easier. Indeed, recent single-cell and single-nuclei transcriptomic studies on human samples of the whole midbrain, the substantia nigra and other associated structures [11,15,34–39], have identified that oligodendrocytes are, after neurons, likely the second most affected cell type in PD (a detailed summary of study results is given in Table 1). Although these datasets largely vary in size, ranging from 17,000 to 316,000 analyzed nuclei, common findings were that oligodendrocytes always comprise the largest cell population in these brain regions and that the transcriptional landscape of oligodendrocytes was always altered. Depending on the detail and focus of the analysis, oligodendrocyte numbers are significantly depleted [39], or shifted in their proportional composition of the subpopulations, with some being depleted, but some also enriched [38]. The identified transcriptional alterations that were identified in PD oligodendrocytes were largely variable across studies, including an increase in stress response [34], inflammation [36], or response to unfolded protein [39] alongside an apparent loss of their myelinating capacity [36,39]. The differences in these findings affecting functional changes could likely be derived from different samples sizes, with largely variable gene numbers and different bioinformatic methods being applied. Most of these studies have combined these data with genome-wide association studies (GWAS) [9,11,35] or other means to include genetic risk-loci [15] and found that the genetic risk in PD is associated not only with dopaminergic neurons, as expected, but also with oligodendrocytes, and that oligodendrocytes can also be associated with clinical outcome prediction [37]. Furthermore, simultaneous use of single-nucleus RNA- and ATAC-sequencing of the human substantia nigra has also revealed that differential changes present in oligodendrocyte transcriptomes can be particularly associated to pathology progression [34].
Particularly surprising in this context is the apparent down regulation of genes involved in myelination, possibly indicating that this particular cell type simply becomes dysfunctional, or that oligodendrocytes need to take on other, as yet unknown, functions [34,36]. This finding is puzzling because dopaminergic neurons in the substantia nigra, the primary cell type affected in PD, are considered to be sparsely myelinated [14], at least according to common knowledge (discussed in the next section). In addition, a stress responding and inflammatory transcriptomic profile has been brought to attention in oligodendrocytes from samples of the striatum and the entire midbrain [34,35]. These results rather suggest a complex functional influence of oligodendrocytes in the pathogenesis of PD that—if oligodendrocytes become affected—goes beyond simple myelin loss and potential cell demise, although clear conclusions are difficult to draw, as human single-cell transcriptomic data come from a single time point of pathology.
In summary, the limited studies on oligodendrocytes in PD provide first evidence of non-cell-autonomous changes associated with reduced functionality of oligodendrocytes that may have widespread effects on the susceptibility of dopaminergic neurons, and therefore raise the hypothesis that these changes may potentially play a role in contributing to disease pathogenesis. While valuable, these studies are largely descriptive and indicate a change in the transcriptional profile of oligodendrocytes. Thus, further studies are needed to shed light on the functional impact of these changes on oligodendrocyte dynamics, and potentially reveal underlying molecular mechanisms contributing to pathology.
Oligodendrocytes in Parkinson’s disease pathology: What remains unknown?
How severely are oligodendrocytes functionally altered in Parkinson’s disease?
Given the discoveries of transcriptional changes in oligodendrocytes in PD highlighted above, several important questions for the field need to be raised. First, do oligodendrocytes play an active role in the pathogenesis of the disease or are they merely secondary victims of the pathological changes in affected neurons? Second, do these cells initiate and promote disease progression in early stages, or do they only respond to global damage from neighboring cells? Literature indeed suggests the possibility of an early active pathogenic role of oligodendrocytes. For example, the transcriptomic studies from human samples reported a shift in their gene expression profile as early as Braak stages 1 and 2 [11]. Nevertheless, these data only represent a single time point in the course of the disease. It is therefore impossible to say what happens in earlier stages of the disease and whether these transcriptomic changes are preceded by other physiological changes. We believe that appropriate human in vitro models could aid address these pathologies and, in long term, could lead to the development of new research strategies and potential therapies in the future.
The ability to combine single-cell transcriptomic and GWAS has enabled researchers to better understand which cell types carry disease-associated single-nucleotide polymorphisms (SNPs) and has contributed tremendously to bringing oligodendrocytes into focus [11]. However, how these SNPs influence the function of oligodendrocytes and in turn disease progression remains to be elucidated. As demonstrated in one study [34], the multi-omics approach that combines single-cell RNA-sequencing with single-cell ATAC-sequenzing (to assess genome-wide chromatin accessibility) on the very same cell, offers the possibility—albeit limited—of understanding which genes are affected by the SNP in a particular cell type. Further development of these combined technologies will greatly improve our understanding of the cell-specific pathological mechanisms in PD. Identifying and understanding these mechanisms requires an enormous collaborative scientific effort, and longitudinal studies using animal models and human patient material would be of great value to the field. This may also answer the question of whether oligodendroglial changes progress linearly or whether the observed pathological alterations also depend on different disease stages. This could open a whole new set of biomarkers that would aid patient care and prognosis.
Does demyelination in the substantia nigra play a role in Parkinson’s disease?
Myelination in patients with PD has been largely assessed by MRI, which, as previously mentioned [12,13], is only an indirect measurement of myelin content; therefore, the question of whether demyelination is a common feature in PD remains largely speculative (Fig 2). Therefore, a thorough examination of the myelin content in patients at different stages of the disease will help us understand whether myelin changes precede and therefore could be causative of neuronal loss, or whether sublethal damaged neurons cause oligodendroglial changes. The general assumption in this field of research is that the degenerating dopaminergic neurons in the substantia nigra are only sparsely myelinated [14], which is probably one reason why the study of oligodendrocytes and myelin has not been the focus of research to date. However, it seems that the low degree of myelination of human dopaminergic neurons has fallen into a state of common knowledge, with actually little experimental evidence to support this statement.
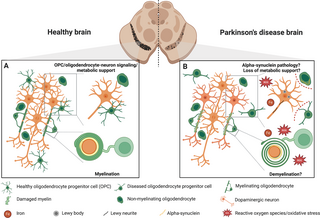
Fig 2. Function and dysfunction of oligodendrocyte lineage cells in healthy and PD brains.
(A) In the healthy brain, the primary role of oligodendrocytes is the myelination of axons, though dopaminergic neurons are generally thought to have minimal myelination [14]. Moreover, both OPCs and non-myelinating oligodendrocytes are recognized for their crucial roles in signaling and providing metabolic support [10], although the exact function of the non-myelinating oligodendrocytes is even less clear. (B) In the PD brain, the particular consequences of damaged or non-functional oligodendrocytes is not well understood. Although there are reports of demyelination in patient brains [12,13], it is still unclear to what extent this affects dopaminergic neuron health or whether demyelination precedes neuronal damage. α-synuclein positive inclusions have been found in oligodendrocytes in PD patient brains [29,30]; however, it remains unclear whether this is due to cell-autonomous build-up of α-synuclein or protein cell-to-cell transfer from neurons to oligodendrocytes. It is hypothesized that diseased oligodendrocytes and OPCs may lose their direct signaling and metabolic support functions (indicated by dashed red lines) to neurons due to pathological changes in their environment, such as the accumulation of ROS and iron (Fe) accumulation that could exacerbate neuronal damage. Created with BioRender.com. OPC, oligodendrocyte progenitor cell; PD, Parkinson’s disease; ROS, reactive oxygen species.
On the contrary, newer studies, showing, for example, high proportions of oligodendrocytes [15,34,35,38] (summarized in Table 2) and high levels of myelination in the substantia nigra [40], suggest a higher degree of myelination of dopaminergic neurons than previously assumed. Additionally, improved technologies have shown that many neurons in regions of gray matter are only intermittently myelinated [41], demonstrating that even small fragments of myelin along axons are critical for complex neuronal functions [42]. Given this updated information, the myelin content of dopaminergic neurons should be reassessed using state-of-the-art technologies. Furthermore, it needs to be considered that PD is characterized by more widespread pathology in other brain regions and involves non-dopaminergic neurons as well. Hence, demyelination and malfunction of other neuronal populations may also contribute to motor and non-motor symptoms associated with disease progression. Finally, the high number of oligodendrocytes in areas with little apparent myelination suggests that myelination is likely not the sole purpose of these cells, and that their contribution to normal brain physiology may be far more pleiotropic than previously thought.
Do oligodendrocyte functions deteriorate due to oxidative stress?
There is strong evidence for an imbalance of brain iron in PD associated with pathology, and the presence of elevated oxidative stress levels is an established pathological feature [45]. Oligodendroglial iron has not yet received much scientific attention in the context of PD, despite oligodendrocytes having the highest iron content in the central nervous system [46], essential for their metabolism. Given the complicated and intimate nature of communication between neurons and oligodendrocytes (Fig 2) and their antioxidant function related to iron metabolism [47], we raise the question whether oligodendrocytes exacerbate or mitigate neuronal iron overload and its associated neurotoxicity. Oligodendrocytes have been shown to be particularly vulnerable to oxidative damage [48], but there is still little known about the sources of reactive oxygen and nitrogen species and the effects on specific functions of oligodendrocytes, particularly with regard to direct coupling of neurons. We speculate that these toxic agents in the environment lead to impaired oligodendrocyte metabolism, rendering them incapable of providing their physiological protection to neurons, or, alternatively, oligodendrocytes themselves could be the source of these toxic components. Therefore, it should be considered that antioxidant support from oligodendrocytes on neurons [47] may still be present, but disease conditions overwhelm the process as the disease progresses.
What is known about the spread of α-synuclein pathology between oligodendrocytes and neurons?
Evidence of oligodendroglial α-synuclein aggregates in PD has recently emerged [29,30] (Fig 2), which was initially thought to be due to oligodendroglial uptake of monomeric and oligomeric forms of α-synuclein [32]. However, more sensitive approaches have revealed that oligodendrocytes do express α-synuclein mRNA and protein [49]; yet the functional and disease-associated implications of this finding remains to be studied. To this end, we propose that advanced disease modeling platforms may be beneficial, such as co-culture systems of neurons with oligodendrocytes, to help assess whether oligodendrocytes are capable of taking up neuronal α-synuclein, thereby reducing the pathological burden, and how this may affect progression of pathology. Or, as another possibility, whether an abnormal function or level of α-synuclein in oligodendrocytes may account as a triggering determinant of pathology affecting α-synuclein levels and accumulation in neighboring neurons. In-depth investigation of this topic is critical to better understand possible risk factors and treatment strategies in PD, as well as other synucleinopathies.
What role might oligodendrocyte progenitor cells play in the pathogenesis of Parkinson’s disease?
Since OPCs were considered merely precursors of oligodendrocytes for a long time after their discovery, many non-canonical functions of OPCs have been discovered, which have been extensively discussed previously [50]. Although not much is known about the contribution of OPCs to PD pathology yet, many of their functions could be of potential importance in disease pathogenesis (Fig 2). Several publications have shown that OPCs are important for neuronal function and their deficiency induces depression-like behavior [51] or obesity [52] in mice. Due to their strong synaptic coupling with dopaminergic neurons [53] (Fig 2), malfunctioning OPCs could disrupt cellular crosstalk, which could, in the long term, lead to functional impairment of this vulnerable cell type. Moreover, OPCs are capable of synapse pruning [54], which may be of pathological significance given the severe synapse loss in PD [55]. Lastly, OPCs have also been shown to have immunomodulatory functions via direct cytokine signaling [56] or through the acquisition of properties of antigen-presenting cells in multiple sclerosis [57,58], a chronic inflammatory condition resulting in oligodendrocyte death and demyelination. With regard to PD, an immunomodulatory function of OPCs could potentially exacerbate neuroinflammation.
Oligodendrocytes in Parkinson’s disease pathology: What are the major challenges?
In recent years, studies using human brain tissue and next-generation transcriptomic approaches have proven indispensable for describing previously unknown pathological changes. However, the tissue is a limited resource and possible artefacts (due to non-standardized handling protocols of the postmortem tissue) is a source of great variability in the data. Furthermore, human brain tissue only provides a snapshot of the disease, and cellular and molecular changes that occurred long before disease onset remain speculative, making additional approaches essential.
Based on the Alzforum database (www.alzforum.org), there are currently 33 genetic and several other drug-induced rodent models of PD available. Although they span over a variety of risk genes, pathological features, and several molecular mechanisms, they all aim at reproducing well-known key pathological hallmarks of the disease and may therefore have limited relevance to oligodendrocyte biology. Moreover, mouse oligodendroglia differ significantly from human oligodendroglia at a fine transcriptomic level [59], which could potentially result in unique species-specific responses to pathological stimuli, further complicating the use of these animal models to study oligodendrocytes. This was indeed shown to be the case in a comparative transcriptomic analysis of human and murine oligodendrocytes in Alzheimer’s disease research [60]. In fact, this study confirmed that the oligodendrocyte activation signature observed in human Alzheimer’s disease is largely distinct from those observed in mice. Possibilities to better understand the pathological mechanisms are, for example, the generation of novel animal modes based on identified molecular pathways that are critical for oligodendrocytes in PD, as well as the “humanization” of animal models through transplantation of human oligodendrocytes.
Human iPSC models have made significant progress, and their use, particularly for studying dopaminergic neurons, has increased significantly in the PD field [8,16]. However, obtaining iPSC-derived oligodendrocytes remains a challenge (see section “Why has the role of oligodendrocytes in PD pathogenesis remained so elusive?”). Although stem cell-derived models do not fully recapitulate the human brain in general, and a neurodegenerative disease due to several limitations in particular, they are highly valuable for studying the underlying molecular mechanisms of cells in isolation. Having robust oligodendrocyte models that can also be kept for an extended amount of time and expanding these to more complex systems, such as co-cultures and oligodendrocyte-harboring and myelinating 3D organoid models, will allow the examination of cell–cell communication and network building. These models should therefore—in parallel to advancing suitable in vivo models—be of highest priority.
Therefore, due to the limitations of all model systems, a concerted scientific approach combining the use of human imaging data and postmortem material, together with human in vitro iPSC platforms and rodent models, may provide some further insight to open scientific questions on this topic.
Conclusions
Oligodendrocytes are a highly abundant cell type in the central nervous system and are essential for brain health and its physiological functions. Therefore, it is not surprising that recent transcriptomic studies have showcased that oligodendrocytes are linked with risk factors of PD and may be involved in the pathogenesis of the disease. Although these studies are extremely interesting and of great value, functional studies are needed to elucidate the role of oligodendrocytes and OPCs in this disease. Studying the causal role of oligodendrocytes, whether they are drivers or bystanders of pathology, the abundance and function of myelin on dopaminergic neurons, the response of oligodendrocytes to oxidative stress, and α-synuclein aggregation appears to be of utmost priority. The full picture requires an orchestrated effort combining the strengths of various in vivo and in vitro models, as well as the use of human brain tissue. Due to their high plasticity, even during adulthood, pharmaceutical targeting of oligodendrocytes in early disease stages could become a valuable therapeutic approach in PD research, with the aim of advancing their neuroprotective potential to slow, stop, or even prevent neurodegeneration in the first place.
Acknowledgments
We thank Rachel Wise and Nadia Dorosti from the ISD for their insightful comments on the manuscript.
References
- 1.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40. pmid:9278044. - 2.
Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23(2):1–13. - 3.
Kamath T, Abdulraouf A, Burris SJ, Langlieb J, Gazestani V, Nadaf NM, et al. Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease. Nat Neurosci. 2022;25(5):588–95. pmid:35513515 - 4.
Okano H, Morimoto S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell. 2022;29(2):189–208. pmid:35120619. - 5.
Chia SJ, Tan E-K, Chao Y-X. Historical Perspective: Models of Parkinson’s Disease. Int J Mol Sci. 2020;21(7). pmid:32252301; PubMed Central PMCID: PMC7177377. - 6.
Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357(6357):1255–61. pmid:28882997; PubMed Central PMCID: PMC6021018. - 7.
Uemura N, Uemura MT, Lo A, Bassil F, Zhang B, Luk KC, et al. Slow Progressive Accumulation of Oligodendroglial Alpha-Synuclein (α-Syn) Pathology in Synthetic α-Syn Fibril-Induced Mouse Models of Synucleinopathy. J Neuropathol Exp Neurol. 2019;78(10):877–90. pmid:31504665; PubMed Central PMCID: PMC6934438. - 8.
Kriks S, Shim J-W, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480(7378):547–51. pmid:22056989; PubMed Central PMCID: PMC3245796. - 9.
Dehestani M, Kessler W, Karmali N, Sun W, Volos P, Tsitkov S, et al. Single-cell transcriptomic changes in oligodendroglial lineage cells derived from Parkinson’s disease patient-iPSCs with LRRK2-G2019S mutation; 2024. - 10.
Simons M, Nave K-A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb Perspect Biol. 2015;8(1):a020479. pmid:26101081; PubMed Central PMCID: PMC4691794. - 11.
Bryois J, Skene NG, Hansen TF, Kogelman LJA, Watson HJ, Liu Z, et al. Genetic identification of cell types underlying brain complex traits yields insights into the etiology of Parkinson’s disease. Nat Genet. 2020;52(5):482–93. pmid:32341526 - 12.
Dean DC, Sojkova J, Hurley S, Kecskemeti S, Okonkwo O, Bendlin BB, et al. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS ONE. 2016;11(10):e0163774. pmid:27706215; PubMed Central PMCID: PMC5051727. - 13.
Boshkovski T, Cohen-Adad J, Misic B, Arnulf I, Corvol J-C, Vidailhet M, et al. The Myelin-Weighted Connectome in Parkinson’s Disease. Mov Disord. 2022;37(4):724–33. pmid:34936123; PubMed Central PMCID: PMC9303520. - 14.
Braak H, Del Tredici K. Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol Aging. 2004;25(1):19–23. pmid:14675725. - 15.
Wang Q, Wang M, Choi I, Sarrafha L, Liang M, Ho L, et al. Molecular profiling of human substantia nigra identifies diverse neuron types associated with vulnerability in Parkinson’s disease. Sci Adv. 2024;10(2):eadi8287. pmid:38198537; PubMed Central PMCID: PMC10780895. - 16.
Kim TW, Piao J, Koo SY, Kriks S, Chung SY, Betel D, et al. Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from hESCs for Translational Use. Cell Stem Cell. 2021;28(2):343–355.e5. pmid:33545081; PubMed Central PMCID: PMC8006469. - 17.
Lee SW, Oh YM, Victor MB, Yang Y, Chen S, Strunilin I, et al. Longitudinal modeling of human neuronal aging reveals the contribution of the RCAN1–TFEB pathway to Huntington’s disease neurodegeneration. Nature Aging. 2024;4(1):95–109. pmid:38066314 - 18.
Sun Z, Kwon J-S, Ren Y, Chen S, Walker CK, Lu X, et al. Modeling late-onset Alzheimer’s disease neuropathology via direct neuronal reprogramming. Science. 2024;385(6708):adl2992. pmid:39088624. - 19.
Bergles DE, Richardson WD. Oligodendrocyte Development and Plasticity. Cold Spring Harb Perspect Biol. 2015;8(2):a020453. pmid:26492571; PubMed Central PMCID: PMC4743079. - 20.
Livesey MR, Magnani D, Cleary EM, Vasistha NA, James OT, Selvaraj BT, et al. Maturation and electrophysiological properties of human pluripotent stem cell-derived oligodendrocytes. Stem Cells. 2016;34(4):1040–53. pmid:26763608; PubMed Central PMCID: PMC4840312. - 21.
Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12(2):252–64. pmid:23395447; PubMed Central PMCID: PMC3700553. - 22.
Ehrlich M, Mozafari S, Glatza M, Starost L, Velychko S, Hallmann A-L, et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc Natl Acad Sci U S A. 2017;114(11):E2243–E2252. pmid:28246330; PubMed Central PMCID: PMC5358375. - 23.
García-León JA, Kumar M, Boon R, Chau D, One J, Wolfs E, et al. SOX10 Single Transcription Factor-Based Fast and Efficient Generation of Oligodendrocytes from Human Pluripotent Stem Cells. Stem Cell Rep. 2018;10(2):655–72. pmid:29337119; PubMed Central PMCID: PMC5830935. - 24.
James OG, Selvaraj BT, Magnani D, Burr K, Connick P, Barton SK, et al. iPSC-derived myelinoids to study myelin biology of humans. Dev Cell. 2021;56(9):1346–1358.e6. pmid:33945785; PubMed Central PMCID: PMC8098746. - 25.
Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, et al. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 2019;22(3):484–91. pmid:30692691; PubMed Central PMCID: PMC6788758. - 26.
Orimo S, Uchihara T, Kanazawa T, Itoh Y, Wakabayashi K, Kakita A, et al. Unmyelinated axons are more vulnerable to degeneration than myelinated axons of the cardiac nerve in Parkinson’s disease. Neuropathol Appl Neurobiol. 2011;37(7):791–802. pmid:21696416. - 27.
Spaas J, van Veggel L, Schepers M, Tiane A, van Horssen J, Wilson DM, et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell Mol Life Sci. 2021;78(10):4615–37. pmid:33751149; PubMed Central PMCID: PMC8195802. - 28.
Thorburne SK, Juurlink BH. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J Neurochem. 1996;67(3):1014–22. pmid:8752107. - 29.
Arai T, Uéda K, Ikeda K, Akiyama H, Haga C, Kondo H, et al. Argyrophilic glial inclusions in the midbrain of patients with Parkinson’s disease and diffuse Lewy body disease are immunopositive for NACP/alpha-synuclein. Neurosci Lett. 1999;259(2):83–6. pmid:10025563. - 30.
Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/α-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol. 2000;99(1):14–20. - 31.
Macnair W, Calini D, Agirre E, Bryois J, Jäkel S, Kukanja P, et al. Single nuclei RNAseq stratifies multiple sclerosis patients into distinct white matter glial responses; 2022. - 32.
Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E. Alpha-synuclein transfers from neurons to oligodendrocytes. Glia. 2014;62(3):387–98. pmid:24382629. - 33.
Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64(3):239–46. pmid:18825660. - 34.
Adams L, Song MK, Yuen S, Tanaka Y, Kim Y-S. A single-nuclei paired multiomic analysis of the human midbrain reveals age- and Parkinson’s disease–associated glial changes. Nature Aging. 2024;4(3):364–78. pmid:38491288 - 35.
Agarwal D, Sandor C, Volpato V, Caffrey TM, Monzón-Sandoval J, Bowden R, et al. A single-cell atlas of the human substantia nigra reveals cell-specific pathways associated with neurological disorders. Nat Commun. 2020;11(1):4183. pmid:32826893 - 36.
Bae E-J, Pérez-Acuña D, Rhee KH, Lee S-J. Changes in oligodendroglial subpopulations in Parkinson’s disease. Mol Brain. 2023;16(1):65. pmid:37710343 - 37.
Dehestani M, Kozareva V, Blauwendraat C, Fraenkel E, Gasser T, Bansal V. Transcriptomic changes in oligodendrocytes and precursor cells associate with clinical outcomes of Parkinson’s disease. Mol Brain. 2024;17(1):56. pmid:39138468 - 38.
Martirosyan A, Ansari R, Pestana F, Hebestreit K, Gasparyan H, Aleksanyan R, et al. Unravelling cell type-specific responses to Parkinson’s Disease at single cell resolution. Mol Neurodegener. 2024;19(1):7. pmid:38245794; PubMed Central PMCID: PMC10799528. - 39.
Smajić S, Prada-Medina CA, Landoulsi Z, Ghelfi J, Delcambre S, Dietrich C, et al. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain. 2022;145(3):964–78. pmid:34919646; PubMed Central PMCID: PMC9050543. - 40.
Walker CK, Roche JK, Sinha V, Roberts RC. Substantia nigra ultrastructural pathology in schizophrenia. Schizophr Res. 2018;197:209–18. pmid:29274737; PubMed Central PMCID: PMC6013319. - 41.
Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, Vercelli A, et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344(6181):319–24. pmid:24744380; PubMed Central PMCID: PMC4122120. - 42.
Bacmeister CM, Huang R, Osso LA, Thornton MA, Conant L, Chavez AR, et al. Motor learning drives dynamic patterns of intermittent myelination on learning-activated axons. Nat Neurosci. 2022;25(10):1300–13. pmid:36180791; PubMed Central PMCID: PMC9651929. - 43.
Braak H, Del Tredici K, Gai WP, Braak E. Alpha-synuclein is not a requisite component of synaptic boutons in the adult human central nervous system. J Chem Neuroanat. 2000;20(3–4):245–52. pmid:11207422. - 44.
Nieuwenhuys R. The greater limbic system, the emotional motor system and the brain. Prog Brain Res. 1996;107:551–80. pmid:8782542. - 45.
Wei Z, Li X, Li X, Liu Q, Cheng Y. Oxidative Stress in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front Mol Neurosci. 2018;11:236. pmid:30026688; PubMed Central PMCID: PMC6041404. - 46.
Todorich B, Pasquini JM, Garcia CI, Paez PM, Connor JR. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467–78. pmid:18837051. - 47.
Mukherjee C, Kling T, Russo B, Miebach K, Kess E, Schifferer M, et al. Oligodendrocytes Provide Antioxidant Defense Function for Neurons by Secreting Ferritin Heavy Chain. Cell Metab. 2020;32(2):259–272.e10. pmid:32531201; PubMed Central PMCID: PMC7116799. - 48.
Giacci MK, Bartlett CA, Smith NM, Iyer KS, Toomey LM, Jiang H, et al. Oligodendroglia Are Particularly Vulnerable to Oxidative Damage after Neurotrauma In Vivo. J Neurosci. 2018;38(29):6491–504. pmid:29915135; PubMed Central PMCID: PMC6705954. - 49.
Mavroeidi P, Arvanitaki F, Karakitsou A-K, Vetsi M, Kloukina I, Zweckstetter M, et al. Endogenous oligodendroglial alpha-synuclein and TPPP/p25α orchestrate alpha-synuclein pathology in experimental multiple system atrophy models. Acta Neuropathol. 2019;138(3):415–41. - 50.
Fang L-P, Bai X. Oligodendrocyte precursor cells: the multitaskers in the brain. Pflugers Arch. 2023;475(9):1035–44. pmid:37401986; PubMed Central PMCID: PMC10409806. - 51.
Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, et al. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron. 2015;88(5):941–56. pmid:26606998; PubMed Central PMCID: PMC5354631. - 52.
Djogo T, Robins SC, Schneider S, Kryzskaya D, Liu X, Mingay A, et al. Adult NG2-Glia Are Required for Median Eminence-Mediated Leptin Sensing and Body Weight Control. Cell Metab. 2016;23(5):797–810. pmid:27166944 - 53.
Caldwell M, Ayo-Jibunoh V, Mendoza JC, Brimblecombe KR, Reynolds LM, Zhu Jiang XY, et al. Axo-glial interactions between midbrain dopamine neurons and oligodendrocyte lineage cells in the anterior corpus callosum. Brain Struct Funct. 2023;228(8):1993–2006. pmid:37668732; PubMed Central PMCID: PMC10516790. - 54.
Auguste YSS, Ferro A, Kahng JA, Xavier AM, Dixon JR, Vrudhula U, et al. Oligodendrocyte precursor cells engulf synapses during circuit remodeling in mice. Nat Neurosci. 2022;25(10):1273–8. pmid:36171430; PubMed Central PMCID: PMC9534756. - 55.
Holmes SE, Honhar P, Tinaz S, Naganawa M, Hilmer AT, Gallezot J-D, et al. Synaptic loss and its association with symptom severity in Parkinson’s disease. NPJ Parkinsons Dis. 2024;10(1):42. pmid:38402233 - 56.
Zhang S-Z, Wang Q-Q, Yang Q-Q, Gu H-Y, Yin Y-Q, Li Y-D, et al. NG2 glia regulate brain innate immunity via TGF-β2/TGFBR2 axis. BMC Med. 2019;17(1):204. pmid:31727112; PubMed Central PMCID: PMC6857135. - 57.
Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat Med. 2018;24(12):1837–44. pmid:30420755; PubMed Central PMCID: PMC6544508. - 58.
Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun. 2019;10(1):3887. pmid:31467299; PubMed Central PMCID: PMC6715717. - 59.
Jäkel S, Agirre E, Mendanha Falcão A, van Bruggen D, Lee KW, Knuesel I, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. 2019;566(7745):543–7. pmid:30747918; PubMed Central PMCID: PMC6544546. - 60.
Pandey S, Shen K, Lee S-H, Shen Y-AA, Wang Y, Otero-García M, et al. Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep. 2022;40(8):111189. pmid:36001972.
ADVERTISEMENT:
Halo, sobat pencinta slot! Pernah denger semboyan “raja slot? jika belum, bersiaplah jatuh cinta dengan konsep ini. slot gaco adalah mesin slot yang selalu kasih kemenangan. Ya, mesin-mesin ini bisa dibilang sebagai andalannya tuk membawa pulang hasil. tapi, cemana sih caranya jumpain raja lot yang benar? Santai Bro, kita beri tenang saja di tempat ini
Games terbaik saat ini hanya satu di Indonesia yaitu yang menyediakan imbal hasil terbesar
Daftarkanlah hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia