Abstract
Neisseria gonorrhoeae is a human-specific pathogen that causes the important sexually transmitted infection, gonorrhoea, an inflammatory condition of the genitourinary tract. The bacterium is closely related to the meningococcus, a leading cause of bacterial meningitis. Both these invasive bacterial species undergo autolysis when in the stationary phase of growth. Autolysis is a form of programmed cell death (PCD) which is part of the life cycle of remarkably few bacteria and poses an evolutionary conundrum as altruistic death provides no obvious benefit for single-celled organisms. Here, we searched for genes present in these 2 invasive species but not in other members of the Neisseria genus. We identified a ~3.4 kb horizontally acquired region, we termed the nap island, which is largely restricted to the gonococcus and meningococcus. The nap island in the gonococcus encodes 3 cationic, bacteriocin-like peptides which have no detectable antimicrobial activity. Instead, the gonococcal Neisseria autolysis peptides (Naps) promote autolytic cell death when bacteria enter the stationary phase of growth. Furthermore, strains lacking the Naps exhibit reduced autolysis in assays of PCD. Expression of Naps is likely to be phase variable, explaining how PCD could have arisen in these important human pathogens. NapC also induces lysis of human cells, so the peptides are likely to have multiple roles during colonisation and disease. The acquisition of the nap island contributed to the emergence of PCD in the gonococcus and meningococcus and potentially to the appearance of invasive disease in Neisseria spp.
Citation: Poncin K, McKeand SA, Lavender H, Kurzyp K, Harrison OB, Roberti A, et al. (2025) Bacteriocin-like peptides encoded by a horizontally acquired island mediate Neisseria gonorrhoeae autolysis. PLoS Biol 23(2):
e3003001.
https://doi.org/10.1371/journal.pbio.3003001
Academic Editor: Sebastian E. Winter, University of California Davis School of Medicine, UNITED STATES OF AMERICA
Received: September 27, 2024; Accepted: January 6, 2025; Published: February 5, 2025
Copyright: © 2025 Poncin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Relevant data are within the paper and its Supporting Information files. Source files for microscopy in Figs 3D and S8 are available at https://doi.org/10.6084/m9.figshare.28077749.v1.
Funding: Wellcome Trust (https://wellcome.org/) awards to CMT (221924/Z/20/Z, 214374/Z/18/Z), and a Long-term postdoctoral scholarship from Wallonia-Brussels International (https://wbi.be/) to KP (A3476). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations:
CE,
Correia element; CFU,
colony-forming unit; HGT,
horizontal gene transfer; IHF,
integration host factor; LB,
lysogeny broth; LDH,
lactate dehydrogenase; MBC,
minimal bactericidal concentration; MDM,
monocyte-derived macrophage; Naps,
Neisseria autolysis peptides; OM,
outer membrane; PCD,
programmed cell death; RBC,
red blood cell; TEM,
transmission electron microscopy
Introduction
Neisseria gonorrhoeae, the gonococcus, causes the sexually transmitted disease, gonorrhoea, a major worldwide public health concern. This human-specific pathogen colonises the mucosal surfaces of the urogenital tract and other sites where it elicits a pronounced inflammatory response [1]. Both N. gonorrhoeae and the closely related pathogen Neisseria meningitidis undergo autolysis, a form of programmed cell death (PCD), as part of their life cycle during the stationary phase of growth [2]. Although autolysis in Neisseria was described over a century ago [3], little is known about the pathways leading to PCD in these species or indeed in any gram-negative bacterium.
Gonococcal autolysis begins with remodelling of peptidoglycan through the activity of enzymes including the lytic transglycosylase, LtgA [4], and subsequent loss of integrity of the outer membrane, through unknown mechanisms, leads to cell death [5]. In contrast, the mechanisms underlying autolysis of Streptococcus pneumoniae are well understood. In this gram-positive invasive pathogen that inhabits the mucosal surface of the upper respiratory tract, activation of pneumococcal LytA, a cell wall-bound amidase, leads to degradation of the peptidoglycan cell wall at teichoic acid-rich areas [6]. Autolysis prevents phagocytosis of S. pneumoniae and liberates the cytotoxin pneumolysin and PAMPs which trigger host cell death and/or responses [7]. Higher rates of PCD in the pneumococcus correlate with the propensity of pneumococcal strains to cause invasive disease [8]. Therefore, PCD could benefit the pneumococcus in vivo by promoting local tissue damage and release of nutrients; however, how suicide evolves in single-celled organisms presents an evolutionary conundrum [9]. Computational studies suggest that PCD in unicellular organisms can only be an adaptive trait if it is a stochastic event [10,11], as in S. pneumoniae where lytA expression is subject to ON:OFF switching through phase variation [12].
To identify genes which associated with invasive potential, we performed comparative genomic analyses of >24,700 Neisseria genomes on PubMLST (https://pubmlst.org/) to genetic elements which are present in the invasive species, N. gonorrhoeae and N. meningitidis, but absent from the other members of the genus. This search revealed a ~3.4 kb horizontally acquired island which we designated the nap island. The nap island is found in N. gonorrhoeae, N. meningitidis, and only a few isolates of the noninvasive species Neisseria bergeri and Neisseria lactamica. In the gonococcus, this region encodes 4 peptides; 3 peptides, NapA, NapB, and NapC are cationic, with features of class II microcins, i.e., size 13]; the GG motif is a signal for cleavage by C39 peptidases prior to secretion [14,15]. The nap island also harbours genes encoding for a putative regulator (NapR), a C39 peptidase (NapP), and an export channel (NapF) related to FapF in Pseudomonas aeruginosa [16]. We show that, distinct from class II microcins secreted by gram-positive bacteria, the gonococcal peptides do not mediate killing of competitor bacterial species. Instead, the N. gonorrhoeae autolysis peptides (Naps) specifically promote PCD of bacteria specifically in the stationary phase of growth but no other stages in their life cycle. Furthermore, mutants lacking Naps display reduced death during stationary phase and reduced autolysis following nutrient deprivation. NapC is also cytotoxic to red blood cells (RBCs), so the acquisition of the nap island may help bacteria shape the local environment at mucosal surfaces in vivo. Genomic analyses suggest that NapR and NapC are phase variable, due to the presence of homo-polymeric nucleotide tracts of different lengths in their open reading frames. This would lead to the stochastic appearance within a clonal population of 2 populations of bacteria, “altruistic” autolytic bacteria and their sibling beneficiaries, indicating that PCD might have evolved in the gonococcus through kin selection. Autolysis would liberate cell contents including nutrients and DNA from bacteria that could be utilised by siblings for growth and genetic diversification, respectively. The pneumococcus, which is a naturally transformable diplococcus, similar to N. gonorrhoeae and N. meningitidis, also displays phase variable PCD [12]. Thus, phase variable PCD may operate at an exquisitely local level with adjacent, non-autolytic, first-degree relatives, i.e., nearest and dearest, the main beneficiaries from dead/dying cells. The secretion of cytotoxic NapC and the release of PAMPs following cell lysis indicate that the acquisition of the nap island and PCD may have been an important step in the emergence of the invasive phenotype in Neisseria spp.
Results
Identification and conservation of the nap island
We searched for pathogen-specific genes in Neisseria spp. to further understand the basis of the invasive phenotype of some species in this genus that usually colonises mucosal surfaces asymptomatically. Using MaGe [17], we identified a ~3.4 kb region in the gonococcal genome, which we designated the nap island (Figs 1A and S1). This region is found in N. gonorrhoeae, with a related locus present in N. meningitidis. However, the nap island is largely absent from noninvasive Neisseria spp. (S2 Fig). The GC content of the nap island (38%) is significantly lower than rest of the genome (53%, Fig 1A), indicating that it was likely acquired by horizontal gene transfer (HGT) from an extant source. Consistent with this, napC, a gene in the centre of the nap island, encodes a protein with 68.9% of identity with a protein from Inoviridae phages (S1 Fig); these phages are associated with Neisseria spp. as 57 Inoviridae sequences are found in Neisseria CRISPR spacers (GenBank accession number DAS81996.1). One end of the island is flanked by napP, encoding a potential C39 peptidase predicted to process GG-containing peptides prior to secretion [14]. At the other end of the island, napF encodes a homologue of FapF, an outer membrane protein in Pseudomonas aeruginosa involved in the secretion of amyloidogenic peptides processed by a C39 peptidase [16]. Of note, both napP and napF have a different GC content compared with the rest of the nap island (respectively, 52% and 50%), suggesting that these 2 genes might have a different origin from the central portion of the island.
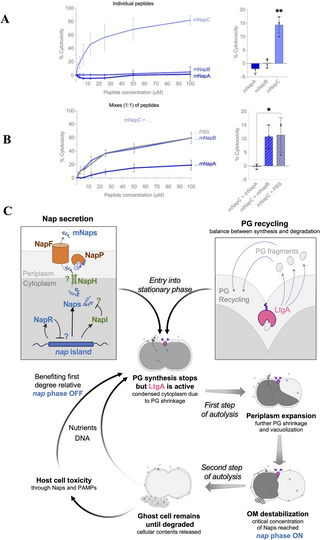
Fig 1. The nap island organisation and the absence of mNaps antimicrobial activity.
(A) Organisation of the nap island in Neisseria gonorrhoeae FA1090. Grey arrows, flanking genes (NEIS0808, pseudouridine synthase; NEIS0794, histidyl-tRNA synthase); blue arrows, genes coding for GG-containing peptides; orange arrows, genes predicted to be involved in peptide processing and secretion; green arrow, gene of unknown function; pink boxes, Correia elements. Percentage of GC content was plotted with Snap Gene Viewer. (B) Table detailing predicted function of genes in the nap locus. Details are available in S1 Fig. (C) Conservation of the nap island and flanking genes among Neisseria spp. Colours are scaled in percent of strain representatives possessing a homologue within each species. The data underlying this figure can be found in S2 Table. (D) mNaps (50 μm) were added to disks which were then placed on lawns of N. gonorrhoeae FA1090 (Ng), N. cinerea (Nc) CCUG 346T or 27178A, L. crispatus (Lc) ATCC 33820, and E. coli (Ec) MG1655 on solid media. Water and polymyxin B (50 μm) were used as negative and positive controls, respectively. Plates were incubated for 24 h and zones of growth inhibition (dotted lines) recorded. No growth inhibition was observed for any mNap (n = 3). Source data is in the file S1 Data. (E) MICs were determined by incubating bacteria in rich medium for 24 h with antibiotics (controls) or mNaps, starting from 256 μg/ml to 0.5 μg/ml in 2-fold dilutions. Bacteria were then spotted onto agar plates, which were incubated for 24 h. MBCs were the lowest concentration at which no growth was visible on plates. The mNaps did not inhibit the growth of any strain (shown by /, n = 3). The data underlying this figure can be found in S1 and S2 Data files.
The gonococcal nap island contains 3 genes (napABC) coding for predicted α-helical cationic peptides with GG-motifs (Fig 1B). After cleavage at the GG sequence, mature mNapA, mNapB, and mNapC are predicted to contain 36, 16, and 31 amino acids, with pIs of 9, 10, and 10.1, respectively (S1 Fig). Downstream of napABC (Figs 1A and S1) is napI which shares 30% of amino acid identity with a domain in LagC, the immunity protein for the bacteriocin, lactococcin G [18] (S1 Fig). napR is upstream of napABC and encodes a peptide with a putative DNA-binding domain (S1 Fig) so is a potential regulator of the island. Finally, napH is predicted to encode an inner membrane protein with a high methionine content with 3 transmembrane α-helices seen in some bacteriophage pore-forming holins [19] (S1 Fig).
Of note, napR contains a poly-G tract of 6 to 12 residues in its 5′ region, indicating that it is likely to be phase variable (S1 Fig and S1 Table). In N. gonorrhoeae FA1090, napR has 7 guanines, the most common allele (in 6,548 out of 10,854 isolates), leading to loss of the GG-leader sequence, so the peptide is predicted to be cytoplasmic. In addition, napC is also probably phase-variable, as it contains a poly-T tract towards its 5′ end resulting typically in a cationic (9xT) or non-cationic (8xT) peptide (S1 Fig and S1 Table).
The distribution of the nap island within Neisseria spp. was examined in more detail using PubMLST [20]. Whole genome sequences of >24,700 Neisseria spp. isolates were inspected, with results confirming that most commensal species lack the nap island with 2 exceptions (Fig 1C). The nap island is found in a proportion of isolates of Neisseria bergeri (38/40 have napA) and Neisseria lactamica (138/604 have napA), but no other noninvasive species (Figs 1C and S2 and S2 Table for details).
Cationic Naps do not inhibit the growth of competitor bacteria
Most class II microcins are antibacterial [21]. To determine whether the cationic Naps possess antibacterial activity, mature versions of the peptides, mNapA, mNapB, and mNapC, were synthesised and their ability to kill competitor bacteria assessed. First, we performed disc diffusion assays against N. gonorrhoeae, Neisseria cinerea, Escherichia coli, and the gram-positive bacterium Lactobacillus crispatus, an inhabitant of the genital tract [22]. As microcins are bactericidal in the nanomolar range [23], assays were performed using discs with 50 μm of each mNap, and with polymyxin B as a positive control. As expected [24], growth of N. cinerea and E. coli was inhibited around discs containing polymyxin B, while there was no clearance of N. gonorrhoeae or L. crispatus around these discs (Fig 1D). In contrast, none of the mNaps inhibited the growth of any strain. We also measured the minimal bactericidal concentration (MBC) of each mNap by broth dilution against the bacteria. Bacteria were incubated with micromolar concentrations of mNaps or polymyxin B for 24 h. Of note, the mNaps failed to inhibit bacterial growth even at the highest concentration (256 μm, Fig 1E).
As some bactericidal mechanisms are contact-dependent [25], we performed co-culture experiments to see whether the survival of prey bacteria (i.e., N. cinerea, E. coli, and L. crispatus) was affected in the presence of wild-type N. gonorrhoeae or an isogenic mutant unable to express Naps. We constructed a markerless N. gonorrhoeae FA1090 ΔnapRABC mutant (eliminating all Nap peptides) with a pheS* counter-selection marker [26] to avoid fitness costs associated with antibiotic resistance cassettes (S3 Fig). After 3 and 24 h of co-culturing prey and N. gonorrhoeae at a 1:1 ratio, we recovered prey strains by plating to selective media. No significant difference could be observed in the recovery of prey strains in the presence of wild-type or ΔnapRABC N. gonorrhoeae (multiple paired t tests, n = 3, S4 Fig). Taken together, these results demonstrate that Naps do not have detectable antimicrobial activity.
Naps mediate N. gonorrhoeae autolysis
To better understand the function of the Naps, we examined the regulation of genes encoding nap island peptides as the expression of many microcins and bacteriocins is regulated during growth [13] (Figs 2A and S5A). The mRNA levels of genes encoding the nap peptides was measured by RT-qPCR of bacteria grown in liquid media over 24 h. The mRNA levels for napA, napB, napC, and napR were lowest in the early stationary phase of growth, then rose to their highest levels in the late autolytic phase. The similar gene expression profile of napA, napB, and napC suggests that they are organised in operon. This is consistent with the absence of a predicted promoter directly upstream of napB (S5B Fig) and the detection of a transcript overlapping all 3 genes in N. gonorrhoeae MS11 [27]. The nap locus of N. gonorrhoeae FA1090 also harbours Correia elements (CEs) in the napR/napA and napI/napH intergenic regions containing potential promoters [28] (Figs 1A and S5B); these CEs harbour integration host factor (IHF) binding sites (S5C Fig), which can influence transcriptional regulation [29].
As nap mRNA levels were elevated during the late stationary phase, we considered whether the Naps are involved in PCD. Gonococcal autolysis involves initial peptidoglycan remodelling by LtgA and other enzymes [4,30], followed by cell lysis. As polyamines can increase the resistance of bacteria to cationic peptides [31,32], we assessed the activity of mNaps on gonococcal survival in protein/spermidine-free media. mNaps were added to N. gonorrhoeae at the mid-log phase, and survival examined during the stationary phase until autolysis occurred [33]. N. gonorrhoeae was grown for 3 h in 96-well plates from a starting OD600 of 0.25, then exposed to mNaps. While mNaps had no effect on survival of gonococci during the exponential phase of growth, micromolar concentrations of mNapA and mNapC significantly reduced survival when bacteria reached the late stationary phase of growth (Fig 2B, p N. cinerea at any stage of growth (S6A Fig), while scrambled versions of mNapA and mNapC (mNapASCR and mNapCSCR, respectively) did not affect survival of N. gonorrhoeae (S6B Fig). We also checked whether mNaps interacted with each other in this assay. Mixtures of Naps were tested on N. gonorrhoeae and results compared with effect of individual mNaps. There was no evidence of antagonism or synergy between the Naps in these conditions (S6C Fig).
Fig 2. Regulation of the nap island and the effect of mNaps on bacterial survival.
(A) RT-qPCR of genes in the nap island from N. gonorrhoeae FA1090 grown in GW liquid cultures. Samples were taken from flasks at different times (CFU, left panel) and mRNA levels of target genes determined by RT-qPCR (right panel). Gene expression results were normalised to samples at T0. (B) Bacteria were grown in 96-well plates in protein-free spermidine-free GW medium for 3 h before mNaps were added at various concentrations (arrow). At time points indicated, bacterial viability was determined by plating to solid media and incubation overnight. CFU were counted (dotted lines, limit of detection). Bottom panels represent the same data but grouped according to the growth phase (exponential and autolytic). Error bars, SD, two-way ANOVA (n = 4; p ≥ 0.033, not significant, not represented; p S3 Data.
As a control for autolysis, we next constructed a markerless N. gonorrhoeae FA1090 ΔltgA mutant; ltgA is involved in the first step of gonococcal autolysis [4]. Importantly, both the ΔnapRABC and ΔltgA mutants displayed increased survival during the late stationary/autolytic phase of growth compared with wild-type bacteria, whether in presence or absence of exogenously added mNaps (Fig 3A, at t, 36 h, p = 0.002, ** for ΔltgA, and p = 0.03, * for ΔnapRABC in the absence of Naps, and p
Fig 3. The nap island is involved in autolysis.
(A) Long-term survival of wild-type N. gonorrhoeae FA1090 and the ΔnapRABC mutant in the presence (arrow) or absence of mNaps (5 μm). The markerless ltgA deletion mutant was a control for a strain with reduced autolysis. Dotted lines, limit of detection. (B) Autolysis in rich medium. Bacteria were streaked on plates, grown overnight then resuspended in GCB at OD540 of ~0.3 and left in cuvettes. After 24 h at room temperature, bacteria were gently resuspended and the OD540 measured. One-way ANOVA with Dunnett’s multiple comparison against the wild-type strain (n = 3; p p p S4 Data. (C) Autolysis in HEPES buffer without (left panel) or with (right panel) 10 mM MgCl2. A representative experiment is shown (last replicate is in the corresponding Suppl. source data files). (D) Transmission electron microscopy images from bacteria in HEPES (T = 75 min). Some wild-type bacteria appear normal (indicated with arrow numbered 1), with many others having a dense (arrow 2) or very dense (arrow 3) cytoplasm, periplasmic expansions (arrow 4), or appearing as ghost cells (arrow 5). Extracellular vesicles (arrow 6) and released cellular content (arrow 7) are also visible. Periplasmic expansions are not observable in the ΔltgA or ΔnapRABC strains. Cytoplasm condensation appears more homogenous in the ΔltgA mutant. The images underlying this figure can be found in https://doi.org/10.6084/m9.figshare.28077749.v1.
To establish whether the nap island directly contributes to PCD, we examined wild-type, ΔnapRABC, and ΔltgA N. gonorrhoeae in 2 independent assays of autolysis; N. cinerea was included as a control. First, we assessed changes in the OD540 of strains incubated overnight in liquid GCB (Fig 3B); a reduction in OD provides a measure of autolysis [34,35]. While the OD540 of wild-type bacteria fell overnight by 34.3% (±4.9, indicating autolysis), the OD540 of the ΔltgA and ΔnapRABC mutants only dropped by 10.8% (±8.9) and 6.5% (±10.6), respectively (Fig 3B, p 540 of N. cinerea increased over this time (Fig 3B, +12.4% ± 4), consistent with the lack of PCD in this species. The rate of autolysis of these strains was also estimated by following the OD540 of bacteria incubated in HEPES, as starvation in this buffer can induce autolysis [5]. Again, the ΔltgA and ΔnapRABC mutants exhibited decreased rates of autolysis compared with wild-type bacteria (Fig 3C), as shown by the turbidity of cultures at the mid-time point (75 min, p p 7A Fig). Autolysis of 2 strains of N. cinerea was also significantly less than N. gonorrhoeae in this assay (Figs 3C and S7C). Consistent with the ability of divalent cations to impair autolysis [34], addition of MgCl2 (final concentration, 10 mM) markedly decreased autolysis (Fig 3C) and abolished any significant difference in autolysis between the wild-type, ΔltgA and ΔnapRABC strains (S7B Fig). To understand the role of NapI during autolysis, we attempted to generate a napI mutant by replacing its open reading frame with a kanamycin resistance cassette. Despite multiple attempts, this was unsuccessful in wild-type bacteria presumably because of the toxicity of Naps in the absence of their immunity protein. Consistent with this, we were able to generate a napI mutant in the ΔnapRABC strain. The napI mutant was incubated in HEPES and the OD540 measured over time. Surprisingly, the ΔnapRABCI mutant was even more resistant to autolysis than the ΔnapRABC strain (S7A Fig), suggesting that NapI might contribute to autolysis or bacterial fitness in absence of other Naps.
Finally, we examined the ultrastructure of the wild-type, ΔltgA, and ΔnapRABC strains by transmission electron microscopy (TEM) after incubation in HEPES buffer to induce autolysis. Before resuspension in HEPES, the strains were indistinguishable with clear nucleoids visible (S8 Fig). After 75 min, wild-type bacteria showed a mix of phenotypes (Fig 3D), some with dense cytoplasm, while other cells displayed an expanded periplasm, or were empty “ghost” cells with evidence of nearby debris. The cytoplasm of the ΔltgA mutant appeared less dense than wild-type bacteria, with no visible periplasmic expansions (Fig 3D). Similarly, the ΔnapRABC mutant lacked periplasmic expansions (Fig 3D), with many cells having aberrant shapes (Fig 3D). Based on the OD540 of bacteria in buffer, both mutants do undergo autolysis. However, TEM images indicate that cell death proceeds differently for the ΔltgA and ΔnapRABC mutants based on differences seen in the expansion of their periplasm. Taken together, our data provide evidence that the Naps contribute directly to autolysis of N. gonorrhoeae.
NapC induces host erythrocyte lysis
As the nap island is largely limited to the gonococcus and meningococcus which can elicit marked inflammatory responses [36], we examined whether the mNaps are toxic to host cells. mNaps were tested for their ability to lyse human RBCs over 1 h at 37°C. Interestingly, mNapC had marked haemolytic activity even at low concentrations (Fig 4A), while neither mNapA or mNapB were toxic. To check for interactions between the peptides, we added combinations of mNaps in a 1:1 ratio to cells. Surprisingly, mNapA protected RBC from lysis by mNapC (Fig 4B). To examine whether mNaps are toxic to other host cells, we also measured the release of lactate dehydrogenase (LDH) by THP-1-derived macrophages following exposure to 1 or 5 μm of each mNap (S9 Fig). No cytotoxicity was detected against THP-1 cells with any mNap, suggesting that their effect is cell-type specific.
Fig 4. Effect of Naps on human red blood cells and proposed model.
(A) Different concentrations of mNaps were added to human RBCs for 1 h and the OD414 measured. Data were normalised to cytotoxicity with melittin (100%), or PBS (0%). Right panel, cytotoxicity induced by 1.56 μm of each mNap (n = 4; p (B) mNapA or mNapB were mixed with mNapC at a 1:1 ratio and cytotoxicity measured. Right panel, results with 3.15 μm of each peptide, n = 3, p S5 Data. (C) Proposed model of gonococcal autolysis. Peptidoglycan (PG) remodelling enzymes, including LtgA (pink) which localises at the septum, will release PG fragments that are recycled (purple arrows). When bacteria reach stationary phase, PG synthesis stops, but LtgA remains active, leading to the condensation of the cytoplasm (Fig 3D). Meanwhile, Naps are processed and secreted by NapP and NapF (orange), respectively, leading to their accumulation in the extracellular environment. Vacuolation is triggered, potentially through the action of inner membrane destabilising actors such as holins/toxins. Eventually, the outer membrane (OM) breaks when a critical concentration of mNaps (blue) is reached, leading to release of cellular contents and the appearance of ghost cells. For diplococci, cells undergoing PCD will benefit first-degree relatives by direct and indirect mechanisms.
Discussion
Many bacteria undergo PCD when subjected to specific environmental stresses such as nutrient deprivation and oxidative damage [37]. This can be mediated by toxin:antitoxin systems, which can also limit the spread of bacteriophage in a population through a process known as abortive infection [37]. In contrast, notably few bacterial species undergo autolysis as a part of their natural life cycle when they enter the stationary phase of growth. Among bacteria with the potential to cause invasive disease, PCD is a feature of the gonococcus, meningococcus, and pneumococcus. These species share the characteristics of being diplococci that are naturally transformable inhabitants of mucosal surfaces in humans. These features are likely to have contributed to autolysis being an adaptive trait in this specific subset of bacteria. We show that the invasive species of Neisseria have acquired a 3.4 kb island which is necessary for their ability to undergo PCD. The success of the nap island as a selfish genetic element is evidenced by its almost ubiquitous distribution in N. gonorrhoeae and N. meningitidis, indicating that the nap island and thence autolysis promotes the success of these bacteria at mucosal surfaces and their invasive potential.
For the gram-positive bacterium S. pneumoniae, the major autolysin LytA is responsible for degradation of cell wall peptidoglycan which is sufficient to cause cell lysis [38]. The situation is more complex for gram-negative bacteria, which possess 2 membranes. Autolysis of pathogenic Neisseria was described over a century ago [3,39]. The first step of gonococcal PCD is peptidoglycan remodelling which depends on the activity of lytic transglycosylases such as LtgA [4,5,30,34]. However, the mechanisms underlying subsequent steps in this process have remained obscure. However, it was known that blocking protein synthesis suppresses autolysis [40] without impeding peptidoglycan hydrolysis [5], suggesting that other proteins are involved in autolysis. Here, we demonstrate that Naps mediate the second step of gonococcal autolysis. We show that addition of mNapA and mNapC reduce bacterial survival specifically during the stationary phase of growth when autolysis occurs. In addition, the napRABC mutant lacking all Naps displayed reduced autolysis compared to the wild-type bacteria. As well as promoting PCD, NapC can be toxic to host RBCs. The peptides are encoded by the nap island, a small horizontally acquired genetic element predominantly found in species of Neisseria which have the capacity to cause invasive disease.
Aside from mediating the release of bacterial PAMPs on death, mNaps might contribute directly to host:pathogen interactions by being toxic to human cells. mNapC can mediate lysis of RBCs at micromolar concentrations; this is counteracted by mNapA, indicating that the interplay of mNaps influence their effect on human cells. In our work, we could not attribute a function to mNapB. One possibility is that NapB does not to regulate autolysis but instead influences other aspects of bacterial life. For example, in S. pneumoniae, competence and fratricide lysis have been functionally linked, since an early competence gene encodes an immunity protein against their own lysins [41]. Since a functional link between the 2 phenomena might also exist in N. gonorrhoeae [42], it might be worth investigating the effect of NapB on competence. Additionally, gram-positive bacteria can also produce multiple bacteriocins; in lactic acid bacteria, the diversity of peptides offers an advantage in distinct environments [43]. Similarly, N. gonorrhoeae might possess multiple Naps to respond to different environmental cues to leading to PCD +/− toxicity of host cells. Considering that some killing mechanisms require direct bacterial contact with target cells [44], it is also possible that gonococcal Naps have further toxic effects on host cells in the presence of bacteria.
Further work is needed to understand the function of the proteins encoded by the nap island. Nevertheless, we propose a model for the role of Naps during autolysis (Fig 4C). N. gonorrhoeae PCD starts with re-modelling of peptidoglycan. Cationic Naps produced in the cytoplasm as immature pre-peptides are cleaved in the periplasm and secreted by NapP and NapF, respectively. The role of the other genes on the nap island is more speculative as they are not always found in nap islands in other species of Neisseria. NapH has features of an inner membrane holin, which allow escape of phages from host cells [45], so might be involved in transporting Naps across the inner membrane. NapR (with a predicted DNA binding domain) could regulate genes on the nap island, while the function of NapI (which has some homology to the immunity protein LagC [18]) could prevent self-intoxication by the cationic Naps.
PCD has several potential benefits to bystander bacteria, such as provision of nutrients [46], enhancing biofilm formation, and the release of DNA to promote genetic diversification of naturally competent bacteria, such as the pathogenic Neisseria. In addition, bacterial lysis could perturb host cells to release nutrients, or alter local inflammatory responses [4]. Lysed RBCs would also release iron, which could help bacteria circumvent nutritional immunity [47]. Importantly, autolysis is also concomitant with the release of bacterial phospholipids [35,48] and peptidoglycan fragments [40,49], which can limit bacterial growth [50] and reduce host innate immune signalling [51], respectively. In S. pneumoniae, the extent of autolysis is correlated with hyper-virulence [8], while the acquisition of horizontally acquired genomic islands in N. meningitidis are associated with invasive capacity of strains [52].
There is a debate over how autolysis evolves in single-celled organisms [37], as PCD is literally a dead-end for a bacterium. Interestingly, NapR and NapC are likely to be phase variable, based on different lengths of homopolymeric tracts in their open reading frames. Based on sequences of over 10,000 gonococcal isolates in PubMLST, napC seems to be mainly OFF (86% of sequenced N. gonorrhoeae strains, n = 7,773/10,854) with an early stop codon preventing the production of the C-terminal cationic amino acids. Phase variation has implications about how the nap island and PCD could be beneficial to a clonal bacterial population [53], and would generate different subpopulations of gonococci, with some bacteria refractory to PCD with other bacteria undergoing altruistic cell death. A bet-hedging strategy, with bacteria switching between 2 distinct phenotypes, could explain how autolysis and altruism evolved in the gonococcus [37]. It is noteworthy that PCD, which is rare among prokaryotes, has evolved through distinct mechanisms in pathogenic diplococci, which are naturally transformable. The release of DNA on autolysis could allow the transfer of beneficial traits between siblings. PCD might be particularly beneficial for diplococci with a dying cell benefitting their first-degree relatives in immediate proximity, i.e., their nearest and dearest (Fig 4C).
In summary, our study sheds light on PCD in invasive Neisseria, a fundamental process relevant for gonococcal cell biology; the nap island encodes NapC which has dual activity, triggering autolysis in addition to death of host cells. As well as the direct effect of the Naps, bacterial suicide might also trigger local and systemic inflammation through the release of PAMPs. Thus, the acquisition of the nap island and its success in N. gonorrhoeae and N. meningitidis might have been an important step in the emergence of these species as invasive pathogens, by enabling them to undergo autolysis to manipulate immune responses and the local environment.
Materials and methods
Bacterial strains and growth
Bacterial strains used in this study are listed in S3 Table. N. gonorrhoeae and N. cinerea were grown on GCB agar plates (1.5% wt./vol. proteose peptone number 3, Becton Dickinson, 0.1% starch, 0.4% K2HPO4, 0.1% KH2PO4, 0.5% NaCl, 1% Vitox, Oxoid, 1.5% agar Oxoid) [54] at 37°C with 5% CO2. L. crispatus was grown on MRS agar plates (ATCC medium 416) and incubated at 37°C with 5% CO2 for about 36 h. E. coli was grown on lysogeny broth (LB) agar plates at 37°C.
Generation of deletion mutants
All primers are shown in S3 Table. For allelic replacement, overlap PCR was performed to obtain resistance cassettes (aph(3)-I or ermC, for kanamycin or erythromycin resistance, respectively) flanked by regions (approximately 1,000 bp) surrounding the target gene. Briefly, overlap PCR consisted in mixing 3 PCR products (“upstream PCR 1” including the START codon of the targeted gene; “downstream PCR 2” including the STOP codon of the targeted gene; “resistance cassette PCR 3”) in equal ratios. For markerless deletions, 2 distinct overlap PCR products were generated: (a) a PCR product consisting of homologous regions (“upstream PCR 1” and “downstream PCR 2”) flanking selection and counterselection cassettes controlled by a constitutive promoter (“popaB-kanR-pheS* PCR 3”, S3 Fig); (b) a markerless PCR product consisting of homologous regions directly bound to each other (“upstream PCR 1” and “downstream PCR 2”). N. gonorrhoeae was transformed as previously [54]. For selection erythromycin (0.5 μg ml−1), kanamycin (80 μg ml−1), or 4CP (8 mM) were added to media. Individual transformants were screened by PCR and confirmed by sequencing.
Bioinformatic analysis
The nap island was identified by MaGe [17] by comparing synteny maps between the reference N. gonorrhoeae FA1090 and N. gonorrhoeae NCCP11945, N. gonorrhoeae FA6140, N. gonorrhoeae 35/02, N. gonorrhoeae PID24-1, N. meningitidis 053442, N. meningitidis FAM18, N. meningitidis MC58, Neisseria lactamica ATCC 23970, N. cinerea ATCC 14685, Neisseria flavescens NRL30031/H210, N. flavescens SK114, Neisseria subflava NJ9703, Neisseria sicca ATCC 29256 and Neisseria mucosa ATCC 25996. Using PubMLST [20], the ORF loci and alleles associated to the nap island were manually curated, as described in https://bigsdb.readthedocs.io/en/latest/curator_guide.html and https://www.youtube.com/watch?v=09g5YdrCtDc. Briefly, using the Neisseria isolates database curator’s interface, the “sequence tags scan” tool allowed us to retrieve alleles in given batches of isolates (see below for isolates selection criteria). New alleles were then validated through sequence alignment and added to the database using Neisseria typing database curator’s interface, “sequences (batch) add” tool. This process was repeated until at least 93.5% of the selected isolates had an allele number attributed to each nap gene. To determine gene conservation (Fig 1C), manual strain selection from the isolate collection of each species on PubMLST was done using the following criteria: a complete rMLST to confirm the species, and the number of contigs S2 Table.
Reverse transcription followed by quantitative PCR
Bacteria were grown in 50 ml of protein- and spermidine-free GW medium [55] in vented flasks (Corning, 431144), by inoculation at OD600nm 0.025 from bacteria grown on GCB plates (T0). RNA was purified from 2 ml of culture pelleted for 4 min at 4,500 rpm and resuspended in TRIzol for 5 min then frozen at −80°C. Tubes were then thawed on ice and mixed with 200 μl of chloroform by vigorous shaking of tubes. After 2 to 3 min incubation at room temperature, tubes were centrifuged at 4°C for 15 min at 12,000 x g and the aqueous phase transferred to 500 μl of ice-cold isopropanol. Tubes were incubated overnight at −20°C, then centrifuged at 4°C for 30 min at 20,000 x g, and pellets were washed with 75% ice cold ethanol, before being resuspended in 80 μl diethylpyrocarbonate-treated H2O. Samples were treated with ezDNase (Invitrogen) and first strand cDNA synthesis was performed with SuperScript IV reverse transcriptase (RT) (Invitrogen) according to manufacturer’s instructions. Note that to obtain specific cDNA, reverse primers (available in S3 Table) were used (alongside the one for the housekeeping gene recA), and a no-RT control was done in parallel. Samples were treated with RNase H for 20 min at 37°C before qPCR was performed using SYBR Green PCR master mix (Applied Biosystems). Primer efficiency was evaluated using serial dilutions to generate a standard curve from PCR products (pre-screen) and mixes of cDNA (to reflect real qPCR conditions). The slopes of standard curves and efficiency values for each primer pair was calculated. StepONEPlus real-time PCR software was used to collect RT-qPCR data and the ΔΔCt method [56] was used to analyse the data, using recA Ct values as reference gene and T0 Ct values as reference condition for Fig 2A. Experiments were done with at least 3 biological replicates, each time with duplicate or triplicate technical repeats.
mNap synthesis
mNaps were synthesised and subjected to HPLC/mass spectrometry by Isca Biochemicals (UK). Note that mNapA was acetylated on its N-terminal side to increase stability. Peptides were diluted in dezionized water supplemented with 0.001% trifluoroacetic acid at 10 mg/ml, aliquoted in 10 μl fractions in Protein Lo-bind tubes (Eppendorf), snap frozen with liquid nitrogen, and stocked at −80°C. Each aliquot was only used once and immediately upon thawing. Batches of peptides were not stocked for longer than 6 months to avoid loss of activity.
Disk diffusion and MBC assays
Bacteria were freshly harvested from plates and resuspended in GW medium [55] at 108 bacteria/ml then spread on agar plates. L. crispatus was plated on MRS agar plates, while all other bacteria were plated on GW agar plates, prepared by mixing (50:50) 2×-concentrated liquid GW medium (filtered) with warm autoclaved 2% agar in water. Plates were allowed to dry for 10 to 15 min, then 6 mm Whatman paper disks soaked with 10 μl of peptide (50 μm) were added. Polymyxin B (50 μm) and water were used as controls. Plates were incubated for 24 to 48 h at 37°C and 5% CO2 as required. The efficacy of compounds was assessed by the presence of a zone of growth inhibition around disks.
All bacteria were harvested from plates and resuspended in FB medium [57]. Peptides were prepared in FB medium as 2×-concentrated solutions at 512 μg/ml and 2-fold dilutions (to 10 μg/ml) were prepared in untreated U-bottom polypropylene 96-well plates (Corning, 3879) to 50 μl per well. Penicillin G and polymyxin B were used as controls. Bacteria (50 μl of 105 CFU/ml) were added to each well, then incubated at 37°C with 5% CO2 with shaking at 180 rpm for 24 h, before spotting 10 μl of each well to plates. After 24 h of incubation, MBC values were attributed to the lowest concentration which gave no growth.
Co-culture assays
Bacteria were harvested from plates, resuspended in FB medium [57], and diluted to 105 CFU (colony-forming unit)/ml. Aliquots of 250 μl of prey bacteria were mixed with 250 μl of either wild-type N. gonorrhoeae FA1090, or the ΔnapRABC mutant, or FB medium alone. Cultures were incubated at 37°C with 5% CO2 with shaking at 180 rpm for 3 and 24 h, before plating to selective agar plates; for N. cinerea, E. coli, and L. crispatus, media were GCB plates supplemented with 0.01% Congo Red [58], LB agar plates, and MRS agar plates, respectively. The number of prey bacteria were normalised to control wells without added gonococci.
Growth curves
Bacteria were resuspended in liquid medium at OD600 0.025, and 100 μl added to wells of a flat-bottom 96-well plate (Greiner, 655161), and the OD600 monitored with a plate reader (BMG LabTech) at 37°C in 5 % CO2 with shaking at 200 rpm. Alternatively, bacteria were resuspended in protein-free spermidine-free GW medium at an OD600 of 0.025, then 90 μl added to wells of untreated U-bottom polypropylene plates (Corning, 3879). After 3 h, 10 μl of peptide was added. Bacterial survival was measured by plating on chocolate agar plates with 5% defibrinated horse blood (E&O labs, PP0100).
Autolysis assays
Bacteria were resuspended in 1.2 ml liquid GCB at OD540 of ~0.3, then left in cuvettes for 24 h at room temperature (21°C), gently resuspended by pipetting before the OD540 was measured again.
Additionally, bacteria were grown in liquid GCB (OD600 of ~0.025) for ~4 h to reach mid-exponential growth, pelleted at 4,500 x g for 5 min and resuspended in 700 μl HEPES buffer (50 mM, pH 8.5) to an OD450 of ~0.3 per ml. Aliquots were added to cuvettes prefilled with 300 μl of HEPES buffer (50 mM, pH 8.5). Just after mixing in pre-filled cuvettes, the OD450 was taken (T0, 100%), then again at regular intervals after gentle resuspending. Values are given in percent of initial turbidity.
Transmission electron microscopy
Samples were prepared as when measuring autolysis in buffer. After 75 min, bacteria were recovered from cuvettes and pelleted at 4,500 G for 5 min before being resuspended in fixation buffer (2.5% glutaraldehyde, 2% formaldehyde, 0.1 M PIPES buffer (pH 7.2)) and left at room temperature for 1 h then stored at 4°C. After fixation, samples were extensively washed in buffer then pelleted, embedded in agarose and dissected into ≤1 mm3 cubes. Samples were treated with 50 mM glycine in buffer for 15 min then washed ahead of secondary fixation for 1 h at 4°C in 1% osmium tetroxide and 1.5% potassium ferrocyanide in buffer. Samples were washed extensively in water and stained overnight in 0.5% uranyl acetate (aq.) at 4°C. The following day, samples were washed in water then dehydrated step-wise in a series of 30%, 50%, 70%, 80%, 90%, 95%, and 100% ethanol. The dehydrated samples were incubated in a 25% solution of low viscosity resin (Agar) diluted in ethanol, then in a 50% solution overnight. Samples were further infiltrated in 75% and then extensively in 100% resin ahead of embedding and polymerisation. Sections of 90 nm were cut from polymerised sample blocks using a Leica UC7 ultramicrotome, post-stained with lead citrate and imaged on a Thermo Fisher Tecnai T12 TEM at 120 keV (with Gatan OneView CMOS camera).
Haemolysis assay
Human blood (1 ml; K2EDTA; Cambridge Bioscience, United Kingdom) was washed 3 times with 4 ml PBS prior to centrifugation at 700 × g for 8 min as previously [59]; erythrocytes were pelleted at 1,000 × g for 10 min, and diluted to 0.5% v/v. Peptides were added to 96-well V-bottomed polypropylene plates at a starting concentration of 100 μm. Mellitin (2.5 μm) and PBS were added as positive and negative controls. Erythrocytes were incubated at 37°C for 1 h, pelleted at 1,000 × g for 10 min; the OD415 nm of supernatants (60 μl) was read, and results normalised to the controls.
LDH release assay
THP-1 Dual cells (InvivoGen) were maintained in RPMI1640 medium supplemented with 10% heat inactivated FBS, 25 mM HEPES buffer, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C, 5% CO2. Cells were differentiated to monocyte-derived macrophages (MDMs) in 96 plates (105 cells per well in 200 μl media) using 50 nM of phorbol 12-myristate 13-acetate (PMA, MP Biomedicals) for 3 h, followed by 3 days in complete media. When required, cells were activated with LPS from E. coli O111:B4 (100 ng/ml, Sigma) for 1 h before being exposed to mNaps.
CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) was used to quantify cell death according to the manufacturer’s protocol. After 6 h, 50 μl of culture supernatant was added to an equal volume of Cytotox 96 reagent. Samples were incubated at room temperature protected from light for 30 min. Then, the OD490 using a microplate reader (BMG Labtech PHERAstar FS) and results normalised to controls.
Supporting information
S1 Fig. Supplementary information regarding genes of the nap island in Neisseria gonorrhoeae FA1090.
DNA and amino acid sequences are given for each nap genes, as well as predicted alpha-fold structures and extra information, such as C39 peptidase cleaving sites, phase variable sequences based on available genomic data (details available in S1 Table) or gene alignments. Note that the alignment between napI and the Lactococcin-G immunity protein lagC was performed after the serendipitous observation that NapP was annotated as homolog to the “Lactococcin-G-processing and transport ATP-binding protein” LagD from Lactococcus lactis both in the genome of N. gonorrhoeae FA19 (GenBank accession no. CP012026.1, locus tag: VT05_00181) and N. gonorrhoeae 35/02 (GenBank accession no. CP012028.1, locus tag: WX61_01768).
https://doi.org/10.1371/journal.pbio.3003001.s001
(PDF)
S2 Fig. Nap island organisation in Neisseria meningitidis (Nm) MC58, Nm FAM18, Neisseria lactamica (Nl) 020–06, and Neisseria cinerea (Nc) NCTC10294.
In both Nm and Nl strains, NapF sequences correspond to the long NEIS0907 allele, which is found in another loci in N. gonorrhoeae (Ng) (under the gene name NGO_0166 in Ng FA1090). In Nm, chromosomal rearrangements led to the fusion between the 2 NEIS0907-containing loci. Genes in purple correspond to those homologous to the NGO_0166-containing locus in Ng FA1090. In Nl, the presence of the long NEIS0907 allele correlates with the loss of napA. Regarding napB, due to the presence of an earlier start codon, it is present as a longer allelic form than in Nm or Ng. As for napI, it is slightly shorter in Nl than in Ng. Note that an extra gene is represented in black in the locus of Nl (NLA_13780, function unknown); a homolog sequence exists in Ng FA1090 but the start codon is not present. As shown in Fig 1C, the locus is absent in Nc. Instead, 3 genes of unknown function (light grey) are present between the flanking NEIS0794 and NEIS0808. ⁑, C39 peptidase predicted cleavage site. Note that for NapB in Nl, ⁑ was not positioned as alternative GG-sites might be at play. Sequences were manually annotated on Snap Gene viewer.
https://doi.org/10.1371/journal.pbio.3003001.s002
(PDF)
S3 Fig. Construction and characterisation of deletion mutants in N. gonorrhoeae.
(A) Growth curves in GCB medium. Markerless deletion strains (plain lines, ML) are compared to resistant marker strains (dotted lines, K for kanamycin resistance marker and E for erythromycin resistance marker). Standard deviation are shown in lighter colours (n = 9). The data underlying this figure can be found in S6 Data. (B) Schematic representation of the classical method to generate deletion mutants in N. gonorrhoeae. Briefly, an overlap PCR product is generated from upstream region (PCR 1), downstream region (PCR 2), and resistance cassette marker (PCR 3) amplifications. The Overlap PCR product is then used for natural transformation into N. gonorrhoeae and the mutant strain is selected based on the acquired antibiotic resistance. (C) Schematic representation of the markerless method. Briefly, 2 overlap PCR products are generated from upstream region (PCR 1) and downstream region (PCR 2) as well as, for the first product only, an endogenous promoter (popaB) followed by section/counter-selection markers (PCR 3) amplifications. Note that the pheS* sequence used here was amplified from the genome of N. gonorrhoeae FA1090 itself and point mutations (* = T275S and A318G) were then introduced by overlap PCR. The markerless method consists in first selecting a recombinant colony through kanamycin resistance selection, and secondly, by transforming this colony with markerless overlap PCR product to allow the counter-selection of the pheS* marker.
https://doi.org/10.1371/journal.pbio.3003001.s003
(PDF)
S4 Fig. Co-culture of prey bacteria with N. gonorrhoeae.
Prey bacteria were incubated alongside either wild-type N. gonorrhoeae (WT FA1090, black bars) or the ΔnapRABC strain (blue bars) for 3 or 24 h in FB medium at a 1:1 ratio. Prey bacteria were then recovered selectively on agar plates and CFU/ml were counted. Data were normalised against the recovery of prey bacteria grown without the gonococcus (100%). The data underlying this figure can be found in S7 Data. Multiple paired t test were performed between each pair (WT vs. ΔnapRABC) with no significant difference in their survival (n = 3, error bars, SD).
https://doi.org/10.1371/journal.pbio.3003001.s004
(PDF)
S5 Fig. Gene expression of the nap island in N. gonorrhoeae.
(A) RT-qPCR on all genes of the nap locus of strain FA1090. Samples were recovered from GW liquid cultures in flasks at different time points, and gene expression was normalised based on samples recovered from plates and resuspended in GW (T0). Error bars represent standard deviation from the mean (n = 3–4). One-way ANOVA was performed on data with minimum 2-fold change (dotted lines) compared to T0 (p p p S8 Data. (B) Predicted ribosome binding sites (RBS) and promoter regions (grey arrows) in the nap locus of strain FA1090. RBS were manually annotated based on the presence of a GGA enriched sequence −3 to −10 of an ATG start codon; promoter regions predicted by BPROM (http://softberry.com). (C, D) Pink arrows represent repeats of Correia elements (CE), while blue boxes show integration host factor (IHF) binding sites, as defined previously (10.1016/s0378-1119(01)00725-9 and 10.1016/s0014-5793(02)02882-x), respectively. Annotations were performed with Snap Gene.
https://doi.org/10.1371/journal.pbio.3003001.s005
(PDF)
S6 Fig. Survival assays in the presence of Naps.
(A) Synthetic mature peptides (mNap) were tested against N. cinerea CCUG 346T. (B) Synthetic scrambled versions of the mature peptides (mNapSCR) were tested on N. gonorrhoeae FA1090. Bacteria were cultured in 96-well plates in protein-free spermidine-free GW medium for 3 h before peptides were added at various concentrations (arrow). At specific time points (0, 3, 6, 9, 24, 30, 36 h), one well per condition was emptied and serial diluted before plating on chocolate agar plates and incubation overnight. Colony-forming units (CFUs) were then counted. Dotted lines represent the limit of detection. (C) In order to check whether mNaps had a synergistic or competitive effect, they were tested against N. gonorrhoeae FA1090 with a fixed concentration of 2.5 μm each, alone or mixed. Data shown here represent the differences (in log scale) between t 0 and 9 h (exponential phase of growth) and t 9 and 36 h (autolytic phase) (n = 3). No synergistic or competitive effect was observed for any of the peptides. Note that the standard deviation at t 36 h was larger than usual due to recovery on GCB plates instead of chocolate agar (see Materials and methods). The data underlying this figure can be found in S9 Data.
https://doi.org/10.1371/journal.pbio.3003001.s006
(PDF)
S7 Fig. Autolysis in 50 mM HEPES buffer (pH 8.5).
(A) Against N. gonorrhoeae in plain buffer. The data shown here are the mean values of 8 biological replicates, except for ΔnapRABC napI::kanR (n = 4). Standard deviation are shown in transparent corresponding colours. One-way ANOVA was performed on data from T = 75 min, with Dunnett’s multiple comparison against the WT values (p p p N. gonorrhoeae in buffer supplemented with 10 mM MgCl2 (n = 3). (C) Against N. cinerea in plain buffer (n = 3). (D) Against N. cinerea in buffer supplemented with 10 mM MgCl2 (n = 3). The data underlying this figure can be found in S10 Data.
https://doi.org/10.1371/journal.pbio.3003001.s007
(PDF)
S9 Fig. Cytotoxicity of Naps on THP-1 cells.
LDH release assays were performed in the presence of 1 and 5 μm of each mNap with non-activated or activated THP-1 derived macrophages. No significant cytotoxic activity was detectable for any mNap (one-sample t and Wilcoxon test); error bars, SD of assays performed in triplicate. The data underlying this figure can be found in S11 Data.
https://doi.org/10.1371/journal.pbio.3003001.s009
(PDF)
References
- 1.
Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018;16(4):226–240. pmid:29430011 - 2.
Wollstein M. Biological relationships of Diplococcus intrecallelularis and gonoccus. J Exp Med. 1907;9(5):588–605. - 3.
Flexner S. Contributions to the biology of Diplococcus intracellularis. J Exp Med. 1907;9(2):105–141. pmid:19867078 - 4.
Cloud KA, Dillard JP. A Lytic Transglycosylase of Neisseria gonorrhoeae Is Involved in Peptidoglycan-Derived Cytotoxin Production. Infect Immun. 2002;70(6):2752–2757. pmid:12010959 - 5.
Wegener WS, Hebeler BH, Morse SA. Cell envelope of Neisseria gonorrhoeae: relationship between autolysis in buffer and the hydrolysis of peptidoglycan. Infect Immun. oct 1977;18(1):210–219. pmid:20406 - 6.
Flores-Kim J, Dobihal GS, Bernhardt TG, Rudner DZ. WhyD tailors surface polymers to prevent premature bacteriolysis and direct cell elongation in Streptococcus pneumoniae. Elife. 2022;11:e76392. pmid:35593695 - 7.
Martner A, Skovbjerg S, Paton JC, Wold AE. Streptococcus pneumoniae Autolysis Prevents Phagocytosis and Production of Phagocyte-Activating Cytokines. Infect Immun. 2009;77(9):3826–3837. pmid:19528220 - 8.
Jacques LC, Panagiotou S, Baltazar M, Senghore M, Khandaker S, Xu R, et al. Increased pathogenicity of pneumococcal serotype 1 is driven by rapid autolysis and release of pneumolysin. Nat Commun. 2020;1892:11. pmid:32312961 - 9.
Ramisetty BCM, Sudhakari PA. « Bacterial Programmed Cell Death »: cellular altruism or genetic selfism? FEMS Microbiol Lett. 2020;367(16):fnaa141. pmid:32821912 - 10.
Libby E, Driscoll WW, Ratcliff WC. Programmed cell death can increase the efficacy of microbial bet -hedging. Sci Rep. 2018;8(1):1120. pmid:29348455 - 11.
Vostinar AE, Goldsby HJ, Ofria C. Suicidal selection: Programmed cell death can evolve in unicellular organisms due solely to kin selection. Ecol Evol. 2019;9(16):9129–9136. pmid:31463010 - 12.
Weiser JN, Markiewicz Z, Tuomanen EI, Wani JH. Relationship between phase variation in colony morphology, intrastrain variation in cell wall physiology, and nasopharyngeal colonization by Streptococcus pneumoniae. Infect Immun. 1996;64(6):2240–2245. pmid:8675333 - 13.
Pons AM, Lanneluc I, Cottenceau G, Sable S. New developments in non-post translationally modified microcins. Biochimie. 2002;84(5–6):531–537. pmid:12423797 - 14.
Dirix G, Monsieurs P, Dombrecht B, Daniels R, Marchal K, Vanderleyden J, et al. Peptide signal molecules and bacteriocins in Gram-negative bacteria: a genome-wide in silico screening for peptides containing a double-glycine leader sequence and their cognate transporters. Peptides. 2004;25(9):1425–1440. pmid:15374646 - 15.
Nes IF, Diep DB, Håvarstein LS, Brurberg MB, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek. 1996;70(2–4):113–128. pmid:8879403 - 16.
Rouse SL, Hawthorne WJ, Berry JL, Chorev DS, Ionescu SA, Lambert S, et al. A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat Commun. 2017;8(1):263. pmid:28811582 - 17.
Vallenet D, Labarre L, Rouy Z, Barbe V, Bocs S, Cruveiller S, et al. MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res. 2006;34(1):53–65. pmid:16407324 - 18.
Oppegård C, Emanuelsen L, Thorbek L, Fimland G, Nissen-Meyer J. The lactococcin G immunity protein recognizes specific regions in both peptides constituting the two-peptide bacteriocin lactococcin G. Appl Environ Microbiol. 2010;76(4):1267–1273. pmid:20038710 - 19.
Young R. Bacteriophage holins: deadly diversity. J Mol Microbiol Biotechnol. 2002;4(1):21–36. pmid:11763969 - 20.
Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. pmid:30345391 - 21.
Hancock REW, Falla T, Brown M. Cationic Bactericidal Peptides. In: Poole RK, éditeur. Advances in Microbial Physiology [Internet]. Academic Press; 1995 [cité 2022 Nov 26]. p. 135–75. Disponible sur: https://www.sciencedirect.com/science/article/pii/S0065291108601459 - 22.
Graver MA, Wade JJ. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann Clin Microbiol Antimicrob. 2011;10(1):8. pmid:21329492 - 23.
Duquesne S, Petit V, Peduzzi J, Rebuffat S. Structural and Functional Diversity of Microcins, Gene-Encoded Antibacterial Peptides from Enterobacteria. Microb Physiol. 2007;13(4):200–209. pmid:17827970 - 24.
Knapp JS, Totten PA, Mulks MH, Minshew BH. Characterization of Neisseria cinerea, a nonpathogenic species isolated on Martin-Lewis medium selective for pathogenic Neisseria spp. J Clin Microbiol. 1984;19(1):63–67. pmid:6361062 - 25.
Booth SC, Smith WPJ, Foster KR. The evolution of short- and long-range weapons for bacterial competition. Nat Ecol Evol. 2023;7(12):2080–2091. pmid:38036633 - 26.
Miyazaki K. Molecular engineering of a PheS counterselection marker for improved operating efficiency in Escherichia coli. Biotechniques. 2015;58(2):86–88. pmid:25652032 - 27.
Remmele CW, Xian Y, Albrecht M, Faulstich M, Fraunholz M, Heinrichs E, et al. Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res. 2014;42(16):10579–10595. pmid:25143534 - 28.
Siddique A, Buisine N, Chalmers R. The transposon-like Correia elements encode numerous strong promoters and provide a potential new mechanism for phase variation in the meningococcus. PLoS Genet. 2011;7(1):e1001277. pmid:21283790 - 29.
Rouquette-Loughlin CE, Balthazar JT, Hill SA, Shafer WM. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol Microbiol. 2004;54(3):731–741. pmid:15491363 - 30.
Dillard JP, Seifert HS. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol Microbiol. 1997;25(5):893–901. pmid:9364915 - 31.
Li J, Beuerman R, Verma CS. Mechanism of polyamine induced colistin resistance through electrostatic networks on bacterial outer membranes. Biochim Biophys Acta BBA—Biomembr. 2020;1862(9):183297. pmid:32339485 - 32.
Goytia M, Shafer WM. Polyamines can increase resistance of Neisseria gonorrhoeae to mediators of the innate human host defense. Infect Immun. 2010;78(7):3187–3195. pmid:20439477 - 33.
Morse SA, Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med Soc Exp Biol Med N Y N. 1974;145(4):1418–1421. pmid:4208046 - 34.
Elmros T, Burman LG, Bloom GD. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1976;126(2):969–976. pmid:4438 - 35.
Bos MP, Tefsen B, Voet P, Weynants V, van Putten JPM, Tommassen J. Function of neisserial outer membrane phospholipase a in autolysis and assessment of its vaccine potential. Infect Immun. 2005;73(4):2222–2231. pmid:15784566 - 36.
Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7(4):274–286. pmid:19287450 - 37.
Bayles KW. Bacterial programmed cell death: making sense of a paradox. Nat Rev Microbiol. 2014;12(1):63–69. pmid:24336185 - 38.
Lewis K. Programmed death in bacteria. Microbiol Mol Biol Rev MMBR. 2000;64(3):503–514. pmid:10974124 - 39.
Sturges WS, Rettger LF. Bacterial Autolysis. J Bacteriol. 1922;7(6):551–577. - 40.
Hebeler BH, Young FE. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975;122(2):385–392. pmid:236277 - 41.
Håvarstein LS, Martin B, Johnsborg O, Granadel C, Claverys JP. New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Mol Microbiol. 2006;59(4):1297–1307. pmid:16430701 - 42.
Chan YA, Hackett KT, Dillard JP. The lytic transglycosylases of Neisseria gonorrhoeae. Microb Drug Resist Larchmt N. 2012;18(3):271–279. pmid:22432703 - 43.
Perez RH, Zendo T, Sonomoto K. Multiple bacteriocin production in lactic acid bacteria. J Biosci Bioeng. 2022;134(4):277–287. pmid:35927130 - 44.
Apicella MA, Edwards JL, Ketterer MR, Weiss DS, Zhang Y, Jen FEC, et al. The phospholipase A of Neisseria gonorrhoeae lyses eukaryotic membranes and is necessary for survival in neutrophils and cervical epithelial cells. MBio. 2024;15(10):e02425–e02424. pmid:39324821 - 45.
Holt A, Cahill J, Ramsey J, Martin C, O’Leary C, Moreland R, et al. Phage-encoded cationic antimicrobial peptide required for lysis. J Bacteriol. 2021;204(1):JB0021421. pmid:34339297 - 46.
Zhang YJ, Rubin EJ. Feast or famine: the host–pathogen battle over amino acids. Cell Microbiol. juill 2013;15(7):1079–1087. pmid:23521858 - 47.
Stoudenmire JL, Greenawalt AN, Cornelissen CN. Stealthy microbes: How Neisseria gonorrhoeae hijacks bulwarked iron during infection. Front Cell Infect Microbiol. 2022;12:1017348. pmid:36189345 - 48.
Cacciapuoti AF, Wegener WS, Morse SA. Cell envelope of Neisseria gonorrhoeae: phospholipase activity and its relationship to autolysis. Infect Immun. 1978;20(2):418–420. pmid:27458 - 49.
Harris-Jones TN, Pérez Medina KM, Hackett KT, Schave MA, Klimowicz AK, Schaub RE, et al. Mutation of mltG increases peptidoglycan fragment release, cell size, and antibiotic susceptibility in Neisseria gonorrhoeae. J Bacteriol. 2023;205(12):e00277–e00223. pmid:38038461 - 50.
Walstad DL, Reitz RC, Sparling PF. Growth Inhibition Among Strains of Neisseria gonorrhoeae due to Production of Inhibitory Free Fatty Acids and Lysophosphatidylethanolamine: Absence of Bacteriocins. Infect Immun. 1974;10(3):481–488. pmid:4214772 - 51.
Knilans KJ, Hackett KT, Anderson JE, Weng C, Dillard JP, Duncan JA. Neisseria gonorrhoeae Lytic Transglycosylases LtgA and LtgD Reduce Host Innate Immune Signaling through TLR2 and NOD2. ACS. Infect Dis. 2017;3(9):624–633. pmid:28585815 - 52.
Mullally CA, Mikucki A, Wise MJ, Kahler CM. Modelling evolutionary pathways for commensalism and hypervirulence in Neisseria meningitidis. Microb Genomics. 2021;7(10):000662. pmid:34704920 - 53.
Hill SA, Masters TL, Wachter J. Gonorrhea—an evolving disease of the new millennium. Microb Cell Graz Austria. 2016;3(9):371–389. - 54.
Dillard JP. Genetic Manipulation of Neisseria gonorrhoeae. Curr Protoc Microbiol. 2011;23(1):4A.2.1–4A.2.24. - 55.
Wade JJ, Graver MA. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol Lett. 2007;273(1):35–37. pmid:17559396 - 56.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. pmid:18546601 - 57.
Kuss S, Couto RAS, Evans RM, Lavender H, Tang CC, Compton RG. Versatile Electrochemical Sensing Platform for Bacteria. Anal Chem. 2019;91(7):4317–4322. pmid:30811935 - 58.
Payne SM, Finkelstein RA. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977;18(1):94–98. pmid:409688 - 59.
Oddo A, Hansen PR. Hemolytic Activity of Antimicrobial Peptides. Methods Mol Biol Clifton NJ. 2017;1548:427–435. pmid:28013523
ADVERTISEMENT:
Hello, sobat pengemar slots pernahkah mendengar semboyan “raja slot? Kalau tidak, bersiaplah jatuh cinta dengan program ini. slot gaco adalah mesin slots yang sering kasih win. Ya, slot-slot ini bisa dibilang sebagai andalannya tuk membawa pulang hasil. any way, cemana sih
tekniknya jumpain raja lot yang benar? Santuy Bro, kita beri tenang saja di sini
Games terpercaya saat ini hanya satu di Indonesia hanya di pasti menyediakan imbal hasil terbesar
SEGERA dengan di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia