Abstract
The DAF-2/insulin/insulin-like growth factor signaling (IIS) pathway plays an evolutionarily conserved role in regulating reproductive development, life span, and stress resistance. In Caenorhabditis elegans, DAF-2/IIS signaling is modulated by an extensive array of insulin-like peptides (ILPs) with diverse spatial and temporal expression patterns. However, the release dynamics and specific functions of these ILPs in adapting to different environmental conditions remain poorly understood. Here, we show that the ILP, insulin-3 (INS-3), plays a crucial role in modulating the response to various environmental stressors in C. elegans. ins-3 mutants display increased resistance to heat, oxidative stress, and starvation; however, this advantage is countered by slower reproductive development under favorable conditions. We find that ins-3 expression is downregulated in response to environmental stressors, whereas, the neurohormone tyramine, which is released during the acute flight response, increases ins-3 expression. We show that tyramine induces intestinal calcium (Ca2+) transients through the activation of the TYRA-3 receptor. Our data support a model in which tyramine negatively impacts environmental stress resistance by stimulating the release of INS-3 from the intestine via the activation of a TYRA-3-Gαq-IP3 pathway. The release of INS-3 systemically activates the DAF-2 pathway, resulting in the inhibition of cytoprotective mechanisms mediated by DAF-16/FOXO. These studies offer mechanistic insights into a brain–gut communication pathway that weighs adaptive strategies to respond to acute and long-term stressors.
Citation: Veuthey T, Florman JT, Giunti S, Romussi S, De Rosa MJ, Alkema MJ, et al. (2025) The neurohormone tyramine stimulates the secretion of an insulin-like peptide from the Caenorhabditis elegans intestine to modulate the systemic stress response. PLoS Biol 23(1):
e3002997.
https://doi.org/10.1371/journal.pbio.3002997
Academic Editor: Ursula Jakob, U of Michigan, UNITED STATES OF AMERICA
Received: February 9, 2024; Accepted: December 20, 2024; Published: January 28, 2025
Copyright: © 2025 Veuthey et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All the raw data and code are available on the Open Science Framework platform (https://osf.io/wfgvs/).
Funding: This work was supported by Grants from: -Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PIP No. 11220200101606CO) to DR. -Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina (PIBAA 28720210101169CO) to TV. -NIH R01GM140480 to MJA. -Agencia Nacional de Promoción de la Ciencia y la Tecnología ANPCYT Argentina (PICT 2019-0480) to DR. -Agencia Nacional de Promoción de la Ciencia y la Tecnología ANPCYT Argentina (PICT-2021-I-A-00052) to DR. -Agencia Nacional de Promoción de la Ciencia y la Tecnología ANPCYT Argentina (PICT-2017-0566) to MJDR. -Agencia Nacional de Promoción de la Ciencia y la Tecnología ANPCYT Argentina (PICT-2020-1734) to MJDR. -Agencia Nacional de Promoción de la Ciencia y la Tecnología ANPCYT Argentina (PICT 2018-03164) to TV. -Universidad Nacional Del Sur (PGI: 24/B291) to DR. -Universidad Nacional Del Sur (PGI: 24/B344) to TV. -Universidad Nacional Del Sur (PGI: 24/B261) to MJDR. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations:
cDNA,
complementary DNA; CGC,
Caenorhabditis Genetics Center; DAF,
Abnormal DAuer Formation; DAG,
diacylglycerol; DMP,
defecation motor program; FOXO,
FOrkhead boX O; GFP, green fluorescence protein; HSF-1,
Heat Shock Factor-1; IIS,
insulin/insulin-like growth factor signaling; ILPs,
insulin-like peptides; INS-3,
insulin-3; IP3,
inositol trisphosphate; ITR-1,
inositol trisphosphate receptor 1; L4,
fourth larval stage; NGM,
Nematode Growth Medium; RICAi,
ring interneuron CUV1; RIM, ring interneuron M; RNAi,
RNA interference; ROI,
region of interest; RT-qPCR,
Reverse transcription-quantitative polymerase chain reaction; TDC-1,
tyrosine decarboxylase-1; TYRA-3,
TYRAmine receptor 3; UV1, uterine-vulval cell 1
Introduction
In nature, animals face changing environmental conditions that require adaptive responses to successfully survive and reproduce. These adaptive responses are orchestrated by sophisticated regulatory mechanisms that control a spectrum of physiological adaptations [1,2]. For example, when conditions are optimal, i.e., abundant food resources and favorable temperatures, animals prioritize and accelerate their growth and reproductive development to gain a competitive advantage [3,4]. Conversely, when faced with adverse environmental conditions, such as food deprivation or oxidative stress, animals adopt a different strategy. They reduce their growth and reproductive rates and redirect their energy toward cytoprotection [5,6]. The evolutionarily conserved insulin/insulin-like growth factor signaling (IIS) pathway plays a pivotal role in this strategic plasticity of animals [7–11]. Elevated levels of insulin and insulin-like peptides (ILPs) are associated with cell growth and development [7,12]. In contrast, reduced insulin levels are associated with increased life span and greater resistance to prolonged environmental stressors such as high temperature or oxidation [13–16]. The mechanisms by which the release of ILPs is coordinated in response to environmental conditions remain largely unknown in all animals.
The complexity of mammalian stress physiology makes it challenging to elucidate the fundamental mechanisms underlying the systemic control of cellular defense processes. Genetic studies in the nematode Caenorhabditis elegans have been instrumental in revealing the importance of the conserved IIS pathway in controlling both life span and stress resistance [15,17,18]. Although C. elegans has a single insulin receptor, DAF-2, its genome encodes 40 ILPs that can interact with this receptor [19]. Downregulation of DAF-2 typically extends life span and increases the resistance to environmental stressors [17]. ILPs have been reported to modulate aversive olfactory learning [20], life span, dauer formation [19,21,22], and germ cell proliferation through the IIS pathway [23]. However, mutation or deletion of single insulin genes failed to completely recapitulate the phenotypes observed in daf-2 mutants [24]. Furthermore, the overexpression of each of the 40 insulin genes revealed that these ILPs can act not only as strong agonists, but also as weak agonists, antagonists, or even exhibit pleiotropic functions on the DAF-2/IIS pathway [25]. Therefore, deciphering the specific roles of individual ILPs is challenging. As in most animals, where ILPs act as hormones, in C. elegans, ILPs are secreted into the pseudocoelom, the fluid-filled body cavity, where they act as long-range signaling molecules [7]. Importantly, the mechanisms underlying ILP secretion in C. elegans are similar to those in mammals, and conserved factors such as ASNA-1, HID-1, and GON-1 have been shown to play a role in insullin secretion [26–28].
Under acute life-threatening situations, such as imminent predation or aggression, organisms initiate an energy-demanding, short-lived “flight response” to increase the chances of escape [29–31]. We have previously uncovered a novel brain–gut communication pathway in C. elegans in which neural stress hormones released during the flight response negatively impact health by activating the DAF-2/IIS pathway [32]. The flight response triggers the activation of a single pair of neurons, called ring interneuron M (RIM), that release tyramine [32,33]. Tyramine, the invertebrate analog of adrenaline, coordinates different motor programs of the flight response, allowing the worm to escape from predation [34–37]. However, the downregulation of tyramine signaling is important for coping with environmental stressors when behavioral responses alone fail to alleviate the exposure to a threatening stimulus [32]. Specifically, we found that tyramine activates an adrenergic-like receptor, TYRA-3, in the intestine. TYRA-3 activation subsequently leads to the stimulation of the DAF-2/IIS pathway throughout the body. Systemic activation of the DAF-2/IIS pathway may increase the metabolic rate required for the physical and energy demands of the flight response, but it comes at a cost: it inhibits cytoprotective mechanisms needed to cope with longer-lasting environmental stressors. Thus, tyramine acts as a state-dependent neural signal that controls the switch between acute and long-term stress responses. How tyraminergic activation of TYRA-3 in the intestine leads to the systemic stimulation of the DAF-2 pathway is unclear.
Here, we find that activation of the tyraminergic receptor TYRA-3 increases calcium transients in the intestine and induces the release of the ILP insulin-3 (INS-3). We show that ins-3 mutants are more resistant to environmental stressors such as oxidation, starvation, and heat. In contrast, during the activation of the flight response, the tyramine-induced release of intestinal INS-3 leads the systemic activation of the DAF-2/IIS pathway, which in turn suppresses cytoprotective mechanisms that rely on DAF-16/FOXO. The tyramine-induced activation of the DAF-2/IIS pathway renders animals more vulnerable to environmental stressors. By elucidating the tyramine-mediated secretion of INS-3, we reveal the molecular sequence underlying the detrimental effects of a sustained flight-stress response in C. elegans.
Results
ins-3 mutants are resistant to environmental stressors
The C. elegans genome encodes an extensive family of ILP genes. Many of these ILPs are expressed in the intestine [24]. Our previous work suggested that ILPs released from the intestine may play a key role in modulating the stress response [32]. To test this hypothesis, we used RNA interference (RNAi) to silence the expression of intestinal ILPs which have been reported to act as strong agonists of the DAF-2/IIS signaling pathway [25]. We examined resistance to the oxidizing agent FeSO4 (15 mM, 1 h) in animals treated with ins-3, ins-4, ins-6, ins-32 and daf-28, RNAi. RNAi silencing of ins-3 increased resistance to oxidative stress (Fig 1A). Like ins-3 RNAi silenced animals, null mutants in the ins-3 gene are significantly more resistant to oxidative stress than control animals (Fig 1B). ins-3 mutants are also more resistant to heat stress (35 °C, 4 h) and starvation compared to control animals (Fig 1B and S1 Fig) suggesting that INS-3 is a general inhibitor of the environmental stress response.
Fig 1. The deficiency of the insulin-like peptide (ILP) INS-3 confers enhanced resistance to environmental stressors.
(A) Survival percentages to oxidation (15 mM FeSO4, 1 h) in animals subjected to RNAi-mediated silencing of ILPs that are expressed in the intestine, and were previously identified as potent agonists of the DAF-2 receptor. Mean ± s.e.m. Three independent experiments were performed (n = 3). Each experiment included 60–80 animals per condition. One-way ANOVA, Holm–Sidak’s post hoc test for multiple comparisons vs. empty vector were used. ns, not significant; * p (B) Survival percentages of wild-type and ins-3 null mutant worms exposed to oxidation (15 mM FeSO4, 1 h) and heat stress (35 °C, 4 h). A two-tailed Student’s t test was used. Three independent experiments were performed (n = 3). Each experiment included 40–80 animals per condition * p https://osf.io/wfgvs/.
We found that a Pins-3::GFP transcriptional reporter is predominantly expressed in the intestine and a subset of neurons consistent with previous reports (Fig 2A) [24]. Transgenic expression of ins-3 under the control of its endogenous promoter in an ins-3 null mutant background restored oxidative stress sensitivity to wild-type levels (Fig 2B). To determine where ins-3 acts to modulate the stress response, we performed tissue-specific rescue experiments. Expression of ins-3 in the intestine but not in neurons, completely restored oxidative and heat stress sensitivity of ins-3 null mutants to wild-type levels (Fig 2B). These findings indicate that INS-3 release from the intestine inhibits the animal’s capacity to cope with environmental stressors.
Fig 2. Intestinal INS-3 modulates the stress response.
(A) Expression of Pins-3::GFP reporter in an L4 worm. Scale bar 50 µm. (B) Stress resistance of ins-3 mutant animals expressing ins-3 cDNA driven by Pins-3 (endogenous), Prgef-1 (pan-neuronal) or Pelt-2 (intestinal) promoters upon exposure to oxidative stress (left) or heat stress (right). Mean ± s.e.m. Three to four independent experiments were performed (n = 3−4). Each experiment included 40−80 animals per condition. One-way ANOVA, Holm–Sidak’s post hoc test for multiple comparisons vs. wild type were used in both graphs. Two-tailed Student’s t test was used in both graphs for comparison between ins-3 null mutant and ins-3; Pelt-2::INS-3. ns: not significant, **p p p https://osf.io/wfgvs/.
INS-3 stimulates the DAF-2/IIS signaling pathway
ILP agonists can activate the DAF-2/IIS receptor, resulting in the phosphorylation of DAF-16 and its retention in the cytosol [13]. To investigate whether intestinal INS-3 systemically activates the DAF-2/IIS pathway, we examined the subcellular localization of the transcription factor DAF-16/FOXO using the translational Pdaf-16::DAF-16::GFP reporter. Under basal conditions, DAF-16 was predominantly localized in the cytoplasm in both wild-type and ins-3 null mutant backgrounds (S2 Fig). Following mild stress induction (35 °C, 10 m), ins-3 mutants showed a significantly higher nuclear accumulation of DAF-16 compared to wild-type animals (Fig 3A). This suggests that in ins-3 mutants, DAF-16 is more readily activated in response to stressors. Notably, the tissue-specific rescue of ins-3 in the intestine reduced nuclear DAF-16 accumulation (Fig 3A). In addition, we found that ins-3 mutants produce fewer offspring and exhibits slightly slower development compared to wild-type animals (S3 Fig), consistent with the downregulation of the DAF-2/IIS pathway [38].
Fig 3. INS-3 inhibits environmental stress response through the inactivation of DAF-16/FOXO.
(A) Up: Representative fluorescence images (20×) depicting the localization of DAF-16a/b::GFP after a short heat exposure (35 °C, 10 min) in wild-type, ins-3 null mutants, and intestinal rescue of ins-3 backgrounds. Scale bar, 50 μm. Bottom: Corresponding scatter dot plots with the number of cells with nuclear DAF-16 per animal (normalized to wild-type). Line shows the median. n = 45–50 animals per condition distributed across (3–4) independent experiments were evaluated. One-way ANOVA with Dunn’s multiple comparisons post hoc test was used. **p p (B) Survival percentages of wild-type animals, ins-3 single mutants, daf-2 single mutants, and ins-3;daf-2 double mutants exposed to oxidative (left) and heat stress (right). Results are shown as mean ± s.e.m. Three independent experiments were performed (n = 3). Each experiment included 65–100 animals per condition. *p p (C) Survival percentages of wild-type animals, ins-3 single mutants, daf-16 single mutants, and ins-3;daf-16 double mutants exposed to oxidative (left) and heat stress (right). Results are shown as mean ± s.e.m. Five (n = 5) and six (n = 6) independent experiments were performed for oxidative stress and heat, respectively. Each experiment included 50–100 animals per condition. *p p (D) Oxidation resistance of animals subjected to intestinal RNAi-mediated silencing of daf-16. The VP303 (kbIs7 [nhx-2p::RDE-1 + rol-6(su1006)]) background allows RNAi silencing only in the intestine [55,109]. Results are shown as mean ± s.e.m. Four independent experiments were performed (n = 4). Each experiment included 30–80 worms per condition. **p p p https://osf.io/wfgvs/.
To determine whether the effects of ins-3 on the stress response depend on its ability to activate DAF-2, we analyzed the stress resistance of ins-3;daf-2 double mutants. Since daf-2 null mutants are extremely resistant to stress [15,32], we used harsh stress conditions (35 °C, 7 h for heat stress and 15 mM FeSO4, 3 h for oxidative stress) to detect potential additive effects in the double mutants. The absence of ins-3 does not further enhance the stress resistance of daf-2 mutants, indicating that INS-3 modulates stress resistance through the activation of DAF-2 (Fig 3B).
In sharp contrast to daf-2 mutants, daf-16 mutants exhibit increased sensitivity to heat and oxidative stress compared to wild-type animals [39–42]. ins-3;daf-16 double mutants displayed stress resistance levels similar to daf-16 single mutants (Fig 3C), suggesting that the stress resistance observed in ins-3 mutants depends on DAF-16 activation. Our results support the conclusion that INS-3 activation of DAF-2 inhibits DAF-16 translocation to the nucleus, impairing the resistance to environmental stressors.
Since ins-3 is required in the intestine to modulate stress resistance, we investigated whether the role of daf-16 is also restricted to the intestine. We performed specific RNAi-mediated knockdown of daf-16 in the intestine in both wild-type and ins-3 null mutant backgrounds. We found that stress resistance in ins-3 mutants with intestinal daf-16 knockdown (ins-3;daf-16 RNAiintestine) is lower compared to ins-3 mutants (Fig 3D). Nevertheless, ins-3;daf-16 RNAiintestine animals are more stress resistant than wild-type animals with intestine-specific daf-16 knockdown (daf-16 RNAiintestine) (Fig 3D). This suggests that while intestinal activation of DAF-16 plays a key role in the enhanced stress resistance observed in ins-3 mutants, the systemic activation of DAF-16 in other tissues is required as well.
Activation of DAF-2 affects the expression of many cytoprotective proteins [43]. One such protein is HSP-16.2, which acts as an effector for DAF-16 and Heat Shock Factor-1 (HSF-1) [43–45]. Expression of the transcriptional reporter of hsp-16.2 (Phsp-16.2::GFP) is induced by heat [32,46]. Under basal conditions, hsp-16.2::GFP fluorescence intensity was similar between wild-type and ins-3 mutant animals (S4A Fig). However, after mild heat stress (35 °C, 15 m), hsp-16.2 expression was markedly increased in ins-3 mutants compared to the wild-type (S4 Fig). Intestinal rescue of ins-3 expression decreased hsp-16.2 expression of ins-3 mutants, albeit not to wild-type levels (S4 Fig).
Since hsp-16.2 expression is regulated by both DAF-16 and HSF-1, we performed genetic experiments using the hsf-1(sy441) loss-of-function mutant. Animals were exposed to the same oxidative- (15 mM FeSO4, 1 h) and heat-stress (35 °C, 4 h) conditions under which ins-3 mutants showed increased resistance. Under these conditions, hsf-1(sy441) mutants did not exhibit increased sensitivity to oxidative stress and were even more resistant to heat stress compared to wild-type animals (S5 Fig). Our results are consistent with recent studies using similar heat stress conditions and developmental stages as those used in our study [47]. While we observed a reduction in oxidative stress resistance in ins-3;hsf-1 double mutants compared to ins-3 single mutants, this was not observed under heat stress conditions. In fact, the loss of hsf-1 function did not affect the increased heat resistance of ins-3 mutants (S5 Fig). These results suggest that DAF-16 activation appears to be the primary effector driving the enhanced stress resistance observed in these mutants. Our experiments, however, do not completely exclude a role for HSF-1 in modulating the responses to harsher stress conditions since hsf-1 has been shown to play a critical role in stress resistance under prolonged stress conditions (e.g., thermotolerance after 9 h at 35 °C [48] or oxidative stress after 24 h of exposure to 200 mM paraquat [49]).
ins-3 expression is differently modulated by distinct types of stressors
Exposure to different stressors can exert both positive and negative effects on the expression of ILP genes [24,50]. To investigate whether stressors affect ins-3 expression, we exposed transgenic animals expressing a Pins-3::GFP reporter to oxidative and thermal stressors (Fig 4A, B). Exposure to either the oxidizing agent FeSO4 (1 h) or heat (30 °C, 6 h), resulted in a decrease in green fluorescence protein (GFP) expression in both the intestine and neurons (Fig 4A, B). Consistent with these results, quantitative real-time PCR (qPCR) experiments also revealed lower ins-3 mRNA levels in animals exposed to these stressors (Fig 4C). This decrease in expression upon exposure to these stressors does not appear to be a non-specific effect on protein expression and/or stability, as the expression of another ILP, ins-4, was unaffected in animals exposed to heat and even slightly increased upon oxidative stress (S6 Fig). In contrast to environmental stressors, exposure to exogenous tyramine significantly increases ins-3 mRNA levels and enhances the expression of the transcriptional Pins-3::GFP reporter in the intestine (Fig 4). Since tyramine is released during the flight response [32,33], our data suggest that environmental stressors and the acute flight response have opposing effects on ins-3 expression.
Fig 4. Environmental stressors and tyramine have opposite effects on ins-3 expression.
(A) Representative epifluorescence images (20×) of L4 animals expressing Pins-3::GFP under basal conditions (20 °C on Nematode Growth Media plates seeded with OP50), under oxidative stress (1 mM FeSO4, 2 h) or heat stress (30 °C, 6 h) and in the presence of exogenous tyramine (15 mM). Scale bar 50 µm. (B) Corresponding quantification of fluorescence levels per worm. Scatter dot plot with relative expression of Pins-3::GFP normalized to basal conditions of each independent experiment. Line at the median. n = 50−200 animals per condition (distributed across 3–4 independent experiments). One-way ANOVA and Dunn’s post hoc test vs. basal were used. * p p (C) Log2 fold-changes in ins-3 transcript levels in animals exposed to oxidative stress (1 mM FeSO4, 2 h) or heat stress (30 °C, 6 h) and in the presence of exogenous Tyramine (15 mM). Negative and positive values indicate down- and up-regulation of ins-3, respectively. Fold change was calculated as ΔCt basal conditions/ΔCt test conditions. Results are shown as mean ± s.e.m. n = 4 independent experiments. The data underlying this figure can be found at https://osf.io/wfgvs/.
Tyramine induces calcium transients in the intestine
The release of tyramine during the flight response stimulates the DAF-2/IIS signaling pathway by activating the Gαq-coupled TYRA-3 receptor in the intestine [32]. We hypothesized that the tyraminergic activation of the Gαq signaling stimulates the release of intestinal ILPs. Activation of Gαq stimulates phospholipase C to generate diacylglycerol (DAG) and inositol trisphosphate (IP3), both of which have been shown to enhance insulin secretion in various organisms [51,52]. IP3 triggers Ca2+ release from intracellular stores via the IP3 receptor intracellular storage [53,54]. Previous studies have demonstrated that the C. elegans IP3 receptor, inositol trisphosphate receptor 1 (ITR-1), regulates intestinal Ca2+ oscillations that drive the defecation motor program (DMP) [55,56]. To analyze the molecular mechanisms downstream of TYRA-3 activation, we expressed the Ca2+ indicator GCaMP6 in the intestine and measured Ca2+ transients upon exposure to tyramine. On food, wild-type animals showed rhythmic Ca2+ waves that propagated from the posterior to the anterior end of the intestine every 45−50 s coinciding with the DMP (Fig 5A, S1 Video). Tyramine deficient, tdc-1 mutants and tyra-3 mutants have a regular DMP and normal Ca2+ waves similar to the wild-type (S7 Fig). In the absence of food, Ca2+ waves and defecation events occur only very rarely in wild-type, tdc-1, and tyra-3 animals consistent with previous observations [57]. Wild-type animals that were exposed to exogenous tyramine (30 mM) displayed a dramatic increase in Ca2+ transients in the intestine, even in the absence of food (Fig 5B–D). Strikingly, tyramine also altered intestinal Ca2+ dynamics, often producing high-frequency Ca2+ waves that initiated from multiple locations along the length of the intestine and moved in both anterior and posterior directions (Fig 5B, S2 Video). While, in the absence of food, exogenous tyramine can induce rhythmic Ca2+ waves and defecation behavior (S3 Video), most wild-type animals (53%) displayed high-frequency Ca2+ waves (Fig 5E). Notably, in tyra-3 null mutants, the tyramine-triggered intestinal Ca2+ transients are significantly reduced (Fig 5B–E, S4 Video). Only 16% of tyra-3 mutants displayed high-frequency waves in response to exogenous tyramine. Quadruple mutants of the tyramine receptors lgc-55, ser-2, tyra-2, and tyra-3 were similar to tyra-3 single mutants, with only 13% displaying high-frequency Ca2+ waves in response to exogenous tyramine (S8 Fig). This suggests that TYRA-3 is the main tyramine receptor that stimulates Ca2+ transients in the intestine. The tyramine-induced increase in intestinal Ca2+ transients was largely abolished in itr-1 mutants that lack the IP3 receptor ITR-1 (Fig 5B–E, S5 Video). Our data support the hypothesis that tyraminergic activation of the TYRA-3 receptor induces intestinal Ca2+ transients through the activation of Gαq-IP3 pathway.
Fig 5. Tyramine increases intestinal Ca2+ transients through the activation of TYRA-3.
(A, B) Representative kymographs of intestinal GCaMP fluorescence in freely behaving animals in the absence (A) or presence (B) of 30 mM tyramine. Kymographs are oriented with the anterior of the animal at the top. Insets at the right of each kymograph show an enlarged view of the Ca2+ waves indicated by the white rectangles. The color map values are at the right. a. u., arbitrary units. (C) Quantification of mean fluorescent intensity over 5-min recordings for wild-type, tyra-3, and itr-1 mutants exposed to 0 mM (n = 10–11 animals) or 30 mM (n = 25–39 animals) tyramine. Results are mean ± s.e.m, Two-Way ANOVA with Tukey’s multiple comparison test. ns, not significant. * p p p p (D) Twenty stacked kymographs with an overlaid trace of mean fluorescent intensity (white lines) for wild-type, tyra-3, and itr-1, animals exposed to 30 mM tyramine, including the examples shown in panel B. The color map values for kymographs are shown at the right. a. u., arbitrary units. The y– axis range for each trace is −1,000 to 8,000 arbitrary units. (E) Table reporting the number of animals that exclusively displayed high-frequency Ca2+ waves without rhythmic Ca2+ waves. The data underlying this figure can be found at https://osf.io/wfgvs/.
Tyramine triggers INS-3 release from the intestine
To determine whether tyramine stimulates the intestinal release of INS-3, we generated transgenic animals expressing the translational reporter INS-3::VENUS driven by the intestinal promoter Pges-1. Secreted VENUS-tagged peptides are taken up by the coelomocytes, which are scavenger cells that continually endocytose material secreted into the pseudocoelomic fluid. Fluorescence intensity in the coelomocytes can therefore be used as a proxy for peptide secretion [26,58,59]. Under basal conditions in transgenic Pges-1::INS-3::VENUS animals, we observed faint fluorescence in the intestine and the coelomocytes (Fig 6 and S9 Fig). This observation is consistent with a low tonic release of INS-3 from the intestine. Upon exposure to exogenous tyramine (15 mM), coelomocyte fluorescence intensity increased 2-fold (Fig 6). The increase in coelomocyte fluorescence is not due to a tyramine-induced increase in expression of INS-3::VENUS transgene driven by the Pges-1 promoter, since qPCR showed no elevation in Venus mRNA levels (S10 Fig). Importantly, VENUS accumulation in coelomocytes following tyramine exposure was significantly reduced in tyra-3 null mutants compared to wild-type animals (Fig 6). Therefore, our data indicate that tyraminergic activation of the TYRA-3 receptor stimulates INS-3 secretion from the intestine.
Fig 6. Tyramine triggers INS-3 release from the intestine.
Representative epifluorescence images (40×) of young not gravid adults expressing Pges-1::INS-3::VENUS in wild-type and tyra-3 null mutant background, in the absence or presence of exogenous tyramine (15 mM). Scale bar 25 µm. Fluorescence is observed in the two posterior coelomocytes of worms (inset). Right. Corresponding quantification of fluorescence intensity on the coelomocytes (normalized to wild-type animals without tyramine exposure). Line at the median. n = 35–40 animals per condition distributed across three independent experiments. For conditions with tyramine, a two-tailed Student’s t test vs. the same strain without tyramine was used. * p https://osf.io/wfgvs/.
Tyramine inhibits cytoprotective mechanisms through intestinal INS-3 secretion
Is INS-3 secretion required for the tyraminergic modulation of the stress response? To address this question, we examined the oxidative and thermal stress resistance of ins-3 null mutants in the presence of exogenous tyramine. Unlike wild-type animals, exogenous tyramine did not impair the stress resistance of ins-3 null mutants (Fig 7A). In addition, when we selectively rescued ins-3 expression in the intestine, the negative impact of exogenous tyramine on stress resistance is restored to wild-type levels (Fig 7A).
Fig 7. Tyramine and INS-3 act in the same pathway to modulate stress resistance.
(A) Stress resistance of ins-3 mutant animals expressing an ins-3 cDNA driven by Pins-3 (endogenous) or Pelt-2 (intestinal) promoters upon exposure to oxidative stress (left) or heat stress (right) in the absence or presence of exogenous Tyramine (15 mM). The detrimental effect of exogenous tyramine on stress resistance is only abolished in ins-3 mutant animals. Mean ± s.e.m. Six-fifteen independent experiments were performed (n = 6−15). Each experiment included 25−30 worms per condition. For conditions with tyramine, two-tailed Student’s t test vs. the same strain without tyramine was used. ns, not significant. * p p p (B) Survival percentages of animals exposed to oxidative (left) and heat stress (right). Results are shown as mean ± s.e.m. Three to twelve independent experiments were performed (n = 3−12). Each experiment included 40−50 animals per condition. The stress resistance is not further enhanced in double mutants tdc-1;ins-3 and tyra-3;ins-3 compared to the single mutants. Additionally, the intestinal expression of ins-3 does not restore resistance to wild-type levels in tyramine-deficient backgrounds. One-way ANOVA, Holm–Sidak’s post hoc test vs. wild-type were used (*p p p p ins-3 were used ( ###p ins-3 (ins-3;tdc-1 and ins-3;tyra-3) compared to the corresponding single mutants. ns, not significant. The data underlying this figure can be found at https://osf.io/wfgvs/.
To further investigate the crosstalk between tyramine and INS-3 in modulating the stress response, we examined the resistance of ins-3;tdc-1 and ins-3;tyra-3 double mutants. These double mutants did not show any further enhancement in stress resistance compared to tdc-1, ins-3, or tyra-3 single mutants (Fig 7B). This observation is consistent with the notion that tdc-1, tyra-3, and ins-3 genes act in the same pathway to regulate the environmental stress response. Unlike our observations in ins-3 null mutant backgrounds, the intestinal rescue of ins-3 in ins-3;tdc-1 and ins-3;tyra-3 backgrounds did not impact the stress resistance (Fig 7B). Furthermore, exogenous tyramine failed to decrease stress resistance in the intestinal ins-3 rescue strain in tyra-3 null mutant background (S11 Fig). These findings strongly support the hypothesis that INS-3 acts downstream of tyramine to modulate the stress response.
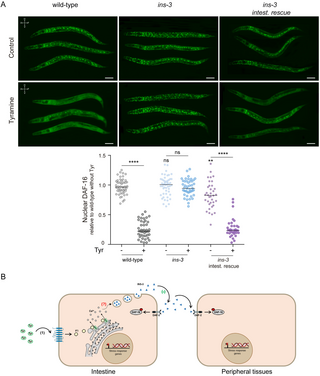
Since nuclear translocation of DAF-16 is enhanced in ins-3 null mutant animals under mild stress (Fig 3A), we analyzed the effects of exogenous tyramine on DAF-16 localization in these mutants. After strong heat (35 °C, 30 min), DAF-16/FOXO predominantly localized to the nucleus in both wild-type and ins-3 mutants [32] (Fig 8A). Exogenous tyramine reduced DAF-16/FOXO nuclear localization in wild-type animals, consistent with previous reports [32]. The addition of exogenous tyramine; however, failed to inhibit DAF-16 nuclear translocation in ins-3 mutant animals (Fig 8A). Intestinal expression of ins-3 restores the inhibitory effect of tyramine on the nuclear translocation of DAF-16/FOXO. This indicates that the negative modulation of DAF-16 by tyramine relies on the release of INS-3 from the intestine (Fig 8A). Taken together, our findings strongly support a model in which the flight neurohormone tyramine activates the TYRA-3 receptor in the intestine, stimulating the release of INS-3 from the intestine; INS-3 in turn, activates the DAF-2 pathway in various tissues, thus inhibiting cytoprotective mechanisms (Fig 8B).
Fig 8. Tyramine inhibits cytoprotective mechanisms through intestinal INS-3 secretion.
(A) Top: DAF-16a/b::GFP localization upon strong heat (35 °C) for 30 min, in the absence or presence of exogenous tyramine (15 mM). Scale bar, 50 μm. Tyramine can inhibit DAF-16 nuclearization upon expression of ins-3 in the intestine. Bottom: Corresponding scatter dot plots with the number of cells with nuclear DAF-16 per animal (normalized to wild-type worms without tyramine; line shows median). n = 35−50 animals per condition distributed across three independent experiments. For conditions without tyramine, One-way ANOVA with Dunn’s multiple comparisons post hoc test vs. the wild type was used. ns, not significant, **p t test (no tyramine vs. tyramine) was used. ns, not significant. ****p (B) Model: The escape neurohormone tyramine activates TYRA-3 in the intestine, elevating calcium levels, likely through IP3-dependent mechanisms involving ITR-1. Activation of TYRA-3 triggers the intestinal release of INS-3, which systemically activates DAF-2. DAF-2 activation results in the phosphorylation of DAF-16, preventing its nuclear translocation and inhibiting the transcription of cytoprotective genes. References: (1) signaling pathway previously found in [32], (?) unknown regions of the pathway; (* ) signaling pathway explained in this article. The data underlying this figure can be found at https://osf.io/wfgvs/.
Discussion
The insulin/insulin-like growth factor signaling (IIS) pathway plays a crucial role in the stress response throughout the animal kingdom [7,60]. Previous studies have shown that downregulation of insulin signaling in C. elegans increases life span, and improves resistance to different environmental stressors [15,18,61–63]. While key components of the IIS pathway, such as the DAF-2 receptor and downstream molecules are well characterized, the specific roles of individual ILPs in the stress response remain largely unclear. The C. elegans genome contains 40 putative ILPs [64], with dynamic temporal and spatial expression patterns with agonist or antagonist impact on the DAF-2 receptor. This complexity indicates the importance of the DAF-2/IIS pathway regulation in the animals’ adaptation to environmental changes. Interestingly, most of the ILPs in the worm are expressed in the intestine [24]. Like other animals, the C. elegans intestine not only serves essential functions in digestion but also plays a pivotal role in the stress response [65]. Previous studies have shown that intestinal DAF-2/IIS is crucial for regulating resistance to oxidative stress, as intestinal-specific deletion of DAF-2 leads to increased oxidative stress resistance in a DAF-16-dependent manner [66]. In addition, the intestine also plays a non-cell autonomous role in the DAF-2-dependent control of life span [67–69].
We find that animals lacking the ILP encoding gene, ins-3, have an increased resistance to oxidative stress, heat, and starvation. Our data show that INS-3 activates the DAF-2/IIS pathway, and thus inhibits the environmental stress response. While ins-3 is expressed in the nervous system and the intestine of C. elegans, intestinal ins-3 expression is sufficient to restore oxidative and heat stress sensitivity to wild-type levels. Our findings indicate that INS-3 release from the intestine plays a key role in modulating systemic environmental stress response, contributing to the broader network of stress regulation in C. elegans. Importantly, the production of ILPs in the intestine may not be unique to C. elegans, as the ability to express and secrete insulin by epithelial cells of the intestine has been reported in rats [70,71]. Furthermore, similar to our observations, this expression appears to be modulated by stressful conditions [72].
While there is increasing recognition of the importance of brain–gut communication in animal physiology and behavior, the molecular mechanisms underlying this interaction remain poorly understood. In previous work, we identified a novel brain–gut communication pathway that sheds light on how the repeated activation of the flight response can impair the reaction to other environmental challenges [32]. Tyramine, considered the counterpart of adrenaline in C. elegans, is synthesized through the decarboxylation of the amino acid tyrosine by the enzyme tyrosine decarboxylase-1 (TDC-1). This enzyme is expressed in the tyraminergic RIM neurons, octopaminergic ring interneuron CUV1 (RIC) neurons, and neuroendocrine uterine-vulval cell 1 (UV1) cells [33]. Despite its expression in these various cell types, we previously demonstrated that tyramine release from RIM neurons during the flight response activates the TYRA-3 receptor in the intestine [32]. TYRA-3 activation, in turn, leads to the systemic stimulation of the DAF-2/IIS pathway and the inhibition of cytoprotective transcription factors, such as DAF-16/FOXO [32]. In this study, we show that the release of tyramine activates TYRA-3, stimulates the secretion of INS-3 from the intestine, and inhibits DAF-2-dependent cytoprotective mechanisms. Phylogenetic analyses indicate that TYRA-3 clusters with other G-protein-coupled receptors that are coupled to Gq signaling pathways [73]. Gq signaling stimulates DAG and IP3 production. We show that tyramine can dramatically increase Ca2+ transients in the intestine of C. elegans, a process modulated by the TYRA-3 receptor and dependent on the IP3 receptor ITR-1. Interestingly, studies in mammals have shown that the activation of Gq receptors and the consequent triggering of DAG- and IP3-dependent mechanisms are essential for insulin secretion [74,75]. DAG activates protein kinase C and opens T-type Ca2+ channels [76]. IP3 stimulates the IP3 receptor, increasing Ca2+ levels from intracellular stores [53,54]. These findings suggest a potential parallelism between the Gq-mediated pathways involved in insulin secretion in mammals and the TYRA-3 signaling pathway in C. elegans. Additional experiments are required to unravel the molecular mechanisms by which activation of a Gq-coupled receptor leads to the release of dense core vesicles containing ILPs. The tyramine-mediated secretion of INS-3, provides a novel molecular brain–gut communication pathway that links the flight response with the suppression of cytoprotective mechanisms.
Although the activation of cytoprotective pathways is crucial to protect against the potential costs of cellular damage, repair, and death, it comes at the expense of resources that could otherwise be allocated to animal development and reproduction [77,78]. This is consistent with our observations that stress-resistant ins-3 mutants, exhibit a slower developmental rate and a reduced brood size. It is likely advantageous for an animal to limit the activation of cytoprotective pathways under favorable conditions, while still retaining the capacity to induce these pathways in emergencies. This delicate balance between maintaining normal reproductive development while preserving inducible cytoprotective capacity is crucial for the survival and overall fitness of an organism. The neural perception of stressors and subsequent regulation of cytoprotective responses play a pivotal role in this plasticity, ensuring that resources are allocated optimally to support both growth and defense mechanisms.
In vertebrates, the release of adrenaline (the vertebrate counterpart of tyramine) in response to acute stressors is a fundamental component of the flight response [79,80]. Frequent exposure to acute stressors can lead to chronic activation of the stress hormone system [81,82]. Similar to our findings in C. elegans, this chronic activation weakens the animal’s defense systems against other stressors, compromising its ability to cope with subsequent challenges [83,84]. In humans, persistent exposure to real or perceived danger, as seen in conditions like stress disorders, has been associated with adverse health outcomes such as type 2 diabetes, increased oxidative stress, neurodegenerative disorders, and premature death [85–90]. Individuals with stress disorders often exhibit hyperinsulinemia [91–94] emphasizing the potential role of insulin dysregulation in stress-related health impairments. The precise mechanisms by which increased levels of stress neurohormones impair general health and defense mechanisms in humans are still not fully understood. However, considering the remarkable conservation of neural control over stress responses across species, it would be interesting to investigate whether chronic release of adrenaline negatively impact health by stimulating the secretion of ILPs.
Materials and methods
Strains and maintenance of C. elegans
Standard C. elegans culture and molecular biology methods were used [95,96]. All C. elegans strains were grown at 20 °C on Nematode Growth Medium (NGM) agar plates with OP50 Escherichia coli as a food source. Low population density was maintained throughout the development and during the assays. The wild-type reference strain used in this study is N2 Bristol. Some of the strains were obtained through the Caenorhabditis Genetics Center (CGC, University of Minnesota), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). All strains used in this study were backcrossed four times with N2 to eliminate possible background mutations.
The strains used were:
- N2 (wild type) [95]
- OAR186 RB1915 ins-3 (ok2488) II 4xBC
- MT10661 tdc-1(n3420) II [33]
- CX11839 tyra-3(ok325) X [97]
- HT1690 unc-119(ed3) III; wwls26 [Pins-3::GFP + unc-119(+)] [24]
- HT1693 unc-119(ed3) III; wwEx63 [Pins-4::GFP + unc-119(+)] [24]
- OAR45 nbaEx8 [Pins-3::INS-3 (20)+lin-15 (80)];ins-3(ok2488), lin-15 (n765ts)]
- OAR43 nbaEx6 [Pelt-2::INS-3 (20)+lin-15 (80)];ins-3(ok2488), lin-15 (n765ts)]
- OAR50 nbaEx13 [Prgef-1::INS-3 (20)+lin-15 (80)];ins-3(ok2488), lin-15 (n765ts)]
- OAR11 tdc-1 (n3420) II; ins-3 (ok2488) II
- OAR169 tyra-3 (n3420) X; ins-3 (ok2488) II
- OAR68 nbaEx6 [Pelt-2::INS-3 (20)+lin-15 (80)]; tyra-3(ok325) X; ins-3 (ok2488) II; lin-15 (n765ts)]
- OAR69 nbaEx6 [Pelt-2::INS-3 (20)+lin-15 (80)]; tdc-1(n3420) II; ins-3 (ok2488) II; lin-15 (n765ts)]
- QW2436 Pges-1::INS-3::VENUS
- TJ356 zIs356[Pdaf-16::DAF-16a/b::GFP + pRF4] [98]
- OAR58 zIs356[Pdaf-16::DAF-16a/b::GFP + pRF4]; ins-3(ok2488) II
- OAR73 zIs356[Pdaf-16::DAF-16a/b::GFP + pRF4]; nbaEx6[Pelt-2::INS-3 (20)+lin-15]; ins-3(ok2488) II; lin-15(n765ts)]
- CL2070 dvIs70[Phsp-16.2::GFP + pRF4] [46]
- OAR100 dvIs70[Phsp-16.2::GFP + pRF4]; ins-3(ok2488) II
- OAR158 dvIs70[Phsp-16.2::GFP + pRF4]; ins-3(ok2488) II; nbaEx6[Pelt-2::INS-3 (20)+lin-15];ins-3(ok2488)II, lin-15(n765ts) X
- CB1370 daf-2(e1370) III [99]
- GR1307 daf-16(mgDf50) I [100]
- PS3551 hsf-1(sy441) I
- OAR56 ins-3 (ok2488) II; daf-2(e1370) III
- OAR99 ins-3 (ok2488) II; daf-16(mgDf50) I
- OAR194 ins-3 (ok2488) II; hsf-1(sy441) I
- VP303 rde-1(ne219) V; kbIs7[nhx-2p::RDE-1 + rol-6(su1006)] [55]
- OAR195 ins-3(ok2488) II; rde-1(ne219) V; kbIs7[nhx-2p::RDE-1 + rol-6(su1006)]
- OAR196 tyra-3(ok325) X; Pges-1::ins-3::venus
- QW2323 zfIs178[Pges-1::NLSwCherry::SL2::GCaMP6s::unc-54 UTR (80ng/ul); lin-15(+)(80ng/ul)] I 4x o.c.
- QW2331 zfIs178[Pges-1::NLSwCherry::SL2::GCaMP6s::unc-54 UTR (80ng/ul); lin-15(+)(80ng/ul)] I; tyra-3(ok325) X
- QW2335 zfIs178[Pges-1::NLSwCherry::SL2::GCaMP6s::unc-54 UTR (80ng/ul); lin-15(+)(80ng/ul)] I; itr-1(sa73) IV
RNAi silencing
RNAi was carried out by feeding nematodes with dsRNA-producing bacteria as described previously [101]. RNAi clones that target C. elegans insulin genes were obtained from Ahringer’s feeding RNAi library [102]. RNAi plates were prepared with standard NGM agar supplemented with 25 mg/ml ampicillin and 1 mM isopropyl b-D-1-thiogalactopyranoside, poured into 35 mm plates and allowed to dry for 7 days at 4 °C. Fresh HT115 E. coli bacteria transformed with the L440 empty vector or carrying the appropriate RNAi clone were grown in 2 mL of LB containing 100 mg/ml ampicillin at 37 °C overnight. Overnight bacterial culture was centrifuged for 2 min at 3,500 rpm and resuspended into 500 µL of LB. Seventy-five microliters of this resuspension were seeded on RNAi plates, and plates were kept overnight at 20 °C for the induction of RNAi.
Animals in the fourth larval stage (L4) were transferred to each plate and maintained at 20 °C. Once they laid eggs, these animals were removed. Therefore, oxidative stress assays were performed in animals (L4 stage) hatched and grown in RNAi plates.
Stress resistance assays
Oxidative stress.
Iron sulfate (FeSO4) was used as an oxidative stressor as previously described [32,103]. Twenty L4 animals were transferred to 35 mm agar plates (4–5 plates) containing FeSO4 at the indicated concentration and time (1 mM, 2 h for microscopy and 15 mM, 1 h for survival assays). To analyze the effect of tyramine on stress resistance, L4 animals were transferred to four 35 mm NGM agar plates (containing 30–40 animals each) seeded with OP50 bacteria with or without 15 mM exogenous tyramine. Stress assays were performed after 14 h.
Heat stress.
Thermotolerance assays were performed as described [32]. For each strain, four 35 mm NGM agar plates containing 20 animals (with or without 15 mM exogenous tyramine) were incubated at 35 °C for 4 h. To ensure proper heat transfer, 6-mm-thick NGM agar plates were used. Animals were synchronized as L4s and used 14 h later. Plates were sealed with Parafilm in zip-lock bags and immersed in a 35 °C water bath for 4 h. After that period, the plates were taken out from the water bath and the zip-lock bags and parafilm were removed. Surviving animals were counted after a 20 h recovery period at 20 °C. For all assays, animals were scored as dead if they failed to respond to prodding with a platinum-wire pick to the nose of the animal.
Starvation resistance.
Food deprivation resistance assays were performed as described [32]. L4 animals were rinsed off the plate, washed with M9 buffer, and then seeded in a 96-well multiwell plate (one worm per well) containing M9. Survival was monitored on days 4, 6, and 8 from the day food was removed. Animals with larvae in their uterus (“bag of worms”) were occasionally observed and were excluded from the quantification.
Microscopy and image analysis
For microscopy, age-synchronized animals were mounted in M9 with levamisole (10 mM) onto slides with 2% agarose pads. ins-3 expression pattern was analyzed by confocal microscopy as previously described [32]. Images were acquired on confocal microscopy (LSCM; Leica DMIRE2) with 20× and 63× objectives. For ins-3 and ins-4 expression levels analysis, animals containing the corresponding transcriptional GFP reporter (Pins-3::GFP and Pins-4:GFP, respectively) were imaged using an epifluorescence microscope (Nikon Eclipse TE2000-5) coupled to a CCD camera (Nikon DS-Qi2) with 20× objective. Fluorescence intensity was quantified using a very simple Macro that runs on Image J FIJI software. With this Macro, the background was initially subtracted, and then a region of interest (ROI) was selected (i.e., in the posterior region of the intestine). The mean fluorescence intensity of this selected region was measured. The instructions for creating this macro are available at OSF (https://osf.io/wfgvs/).
Tyramine supplementation
Tyramine hydrochloride (Alfa Aesar) stocks were made with MilliQ sterile water and diluted to 15 mM into NGM agar before pouring. For calcium imaging experiments, tyramine was diluted to 30 mM in agar. Plates were stored at 4 °C and used within 1 week after pouring.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from approximately 100 adult worms using Bio-Zol RNA isolation reagent (PBL-Productos Bio-Logicos). To lyse the worms, five cycles of cold–warm shock were applied, alternating between liquid nitrogen and a 35 °C water bath. Following RNA extraction, the RNA was reverse-transcribed into complementary DNA (cDNA) using EasyScript Reverse Transcriptase (TransGen Biotech).
Gene expression analysis was conducted using qPCR on a Rotor-Gene 6000 (Corbett Research) with an SYBR Green Master Mix. The cycling conditions for qPCR were set as follows: an initial incubation at 50 °C for 2 min, followed by denaturation at 95 °C for 2 min, and then 45 cycles of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. A melting curve analysis was performed after the final cycle to assess the specificity of the amplified products in each reaction.
The expression level of each gene was quantified relative to that of actin, used as the housekeeping gene control, with ΔCT calculated as ∆ CT = CT Gene of interest − CT actin. The transcript levels of the gene of interest for each condition were assessed relative to the control condition using the formula (log2 = (∆CTcontrol/ ∆ CTTest condition). Positive values indicate an increase in expression levels, while negative values indicate a decrease compared to the control condition. Values of Ct above 35 are below the detection limit and considered background noise. All experiments were performed at least as three independent assays. For each assay, we run three technical replicates of each PCR reaction. Primers were designed using Integrated DNA Technologies online design tools.
Primer sequences for RT-qPCR
- ins-3 (Forward):CCTGATGGCCAGATCAAGAA
- ins-3 (Reverse):ACATTCTCCTCCACACATTACC
- venus (Forward):ACAGCCACAACGTCTATATCAC
- venus (Reverse):TTGTACAGCTCGTCCATGC
- hsp-16.2 (Forward):ACCACTATTTCCGTCCAGCT
- hsp-16.2 (Reverse):GGCGTTCCATCAGAGCCAT
Calcium imaging
Young adult animals expressing the integrated transgene zfIs178[Pges-1::NLSwCherry::SL2::GCaMP6s] were transferred to agar plates containing 0 or 30 mM tyramine and allowed to acclimate for 5 min prior to imaging with a 5× objective (Zeiss, Fluar 5×/0.25) on an inverted fluorescent microscope (Zeiss, Axio observer A1) equipped with an EGFP/mCherry optical splitter (Cairn, OptoSplit II). The Chroma 59022 ET filter set was used in the microscope and Chroma filters ET525/50m, ET632/60m, and dichroic T560lpxr were used in the optical splitter. Transmitted light provided a brightfield image in the mCherry channel that was used for analysis. Recordings were acquired at 15 frames per second with a 66-ms exposure time on an ORCA-Flash4.0 Digital CMOS camera (Hamamatsu, C13440-20CU) using μManager software [104] with the Split View plugin to generate an image stack with interleaved brightfield and GFP channels.
Intestinal calcium measurement
Custom MATLAB (The MathWorks) scripts were used to measure intestinal GCaMP fluorescence. Brightfield images were thresholded using Otsu’s method to generate a binary mask which was refined through morphological erosion and dilation and size filtering. A ROI was generated from the processed binary mask which was used to measure pixel values in the GCaMP channel. A background subtracted mean fluorescent signal was calculated for each frame by subtracting the mean gray value of pixels outside of the ROI from the mean gray value of pixels inside the ROI. To generate kymographs of GCaMP signal, a midline was created via skeletonization of the ROI. At each pixel of the midline, the neighboring pixels were used to create a line segment. The maximum gray value was measured along a line perpendicular to each line segment and the mean background signal was subtracted to yield GCaMP fluorescence along the length of the worm. Linear interpolation was used to ensure a consistent number of midline samples to plot kymographs. Quantification of rhythmic Ca2+ waves and high-frequency ripples was done by visual inspection of the kymographs and mean fluorescent signal traces. Rhythmic Ca2+ waves were identified by two or more peaks that rose above baseline fluctuation in the mean fluorescent signal. High-frequency waves were identified by local oscillations in the kymograph that have a period of less than 30 s. Animals were scored as displaying high-frequency Ca2+ waves if local oscillations were present and rhythmic propagating calcium waves were not detectable.
INS-3 release quantification
A population of animals expressing the Pges-1::INS-3::VENUS transgene was exposed to tyramine (15 mM, 14 h). Venus is resistant to quenching in low pH environments and has been extensively used to monitor peptide secretion from dense core vesicles in C. elegans [105–107]. Individuals were immediately mounted and anesthetized as described above. Images were acquired on confocal microscopy (LSCM; Leica DMIRE2). Images were loaded into ImageJ and the soma of the coelomocytes were identified from their anatomical location. A circular ROI was placed around the coelomocyte to measure the mean fluorescence intensity value of each cell. The values obtained for each condition were normalized to the average intensity of the coelomocytes in the control condition.
Subcellular DAF-16 localization
DAF-16 translocation was analyzed using strains containing the translational Pdaf-16::DAF-16A/B::GFP reporter in a wild type, or insulin mutant background (Pdaf-16::DAF-16/B::GFP;ins-3; Pdaf-16::DAF-16A/B::GFP;ins-3; Pelt-2::INS-3). Young adult, not gravid animals selected after the last molt (at least 25 animals per experiment, repeated 3–4 times) in basal conditions or exposed to either mild- (35 °C, 10 m) or strong-heat stress (35 °C, 30 m), with or without exogenous tyramine, were mounted to analyze DAF-16 cellular distribution under a fluorescence microscope. The number of GFP-labeled nuclei per animal was quantified using Image J FIJI software and normalized to the wild-type strain without tyramine condition within the same day.
Expression analysis of DAF-2–IIS target genes
hsp-16.2 expression levels were analyzed in transgenic strains containing transcriptional reporters in the wild-type and ins-3 mutant backgrounds. Animals were imaged using an epifluorescence microscope (Nikon Eclipse E-600) coupled to a CCD camera (Nikon K2E Apogee). Fluorescence intensity was quantified using Image J FIJI software.
Molecular biology and cloning
Standard molecular biology techniques were employed for the endogenous rescue of ins-3. The entire ins-3 gene, along with a 2.1 kb upstream region from the start codon, was amplified from genomic DNA. Subsequently, this fragment was cloned into a vector backbone derived from the plasmid Ppd95.75, utilizing the unc-54 3’UTR. For the construction of intestinal and neuronal rescue constructs, the ins-3 genomic DNA (including intronic sequences) from this construct was subcloned behind the intestinal reporters elt-2 and the pan-neuronal rgef-1 promoter, respectively. Since we did not observe changes in stress resistance in Prgef-1::INS-3 rescued animals compared to the ins-3 null mutant, we performed qPCR to ensure that ins-3 is effectively expressed (S1 Table). Additionally, the ins-3 gene was subcloned into a plasmid containing the intestinal promoter ges-1 and the YFP variant Venus [58] to generate the reporter Pges-1::INS-3::VENUS. The sequences of the constructed plasmids are available on the Open Science Framework platform (https://osf.io/wfgvs/). Transgenic strains were generated through the microinjection of plasmid DNA at 20 ng/μl into the germline, along with the co-injection marker lin-15 rescuing plasmid pL15EK (80 ng/μl), into lin-15(n765ts) mutant animals. For the intestinal expression of INS-3 and INS-3::VENUS, the plasmid DNA concentration was 3 ng/μl, since higher concentrations did not yield transgenic animals (possibly due to toxic effects of high INS-3 levels in the intestine). At least three independent transgenic lines were established, and the data presented are from a single representative line.
Double mutants were obtained using standard techniques [108]. In brief, 10 males from one of the mutant strains of interest (tdc-1 or tyra-3) were mated with 2 ins-3 null mutant hermaphrodites. After 72 h, F1 worms (one per plate) were isolated to allow for the production of offspring. The resulting F2 generation was isolated again. The double mutants were identified by PCR analysis of genomic DNA.
Data collection and statistics
All data are represented in a format that shows the distribution (dot plots) and all the graph elements (median, error bars, etc.) are defined in each figure legend. For most of our experiments, as is usual in C. elegans research, we used a large number of animals per condition in each assay (typically more than 40–50 animals). This number of animals is large enough to ensure appropriate statistical power in the tests used. All the statistical tests were performed after checking normality. Grubbs’ test was used for outliers analysis (p
Supporting information
S1 Fig.
ins-3 null mutants are more resistant to starvation than wild-type animals.
Survival percentages of wild-type and ins-3 null mutant worms upon 4, 6, and 8 days of starvation. Three independent experiments were performed n = 3. Each experiment included 45–50 animals per condition. Two-tailed Student’s t test was used. * p https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s001
(JPG)
S2 Fig. DAF-16 primarily localizes to the cytoplasm in non-stressed ins-3 null mutants.
Representative fluorescence images (20×) depicting the localization of DAF-16a/b::GFP under basal conditions in wild-type, ins-3 null mutant, and intestinal rescue of ins-3 backgrounds. No nuclear localization of DAF-16 was observed. n = 20–30 animals per condition. Scale bar, 50 μm.
https://doi.org/10.1371/journal.pbio.3002997.s002
(JPG)
S3 Fig.
ins-3 mutants have a reduced brood size and develop more slowly than wild-type animals.
Left. Total number of progeny per worm in wild-type and ins-3 null mutants. n = 25–30 animals per condition distributed across (three) independent experiments. Two-tailed Student’s t test was used. **p ins-3 null mutant worms. A color code was used to represent each of the following animal stages: L1–L3: early larval stages, L4: last larval stage, > L4: adult stage. The animal classification was evaluated at the indicated time points (24, 36, 48, 60, and 72 h). Five to six independent experiments were performed (n = 5–6). Each experiment included 100–200 worms per condition Data are represented in a stacked bar chart as mean. A two-tailed Student’s t test was used. * p https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s003
(JPG)
S4 Fig.
hsp-16.2 expression is enhanced in ins-3 mutants.
(A) Representative fluorescence images (20×) of animals expressing Phsp16.2::GFP in wild-type, ins-3 null mutants, and intestinal rescue of ins-3 backgrounds under basal conditions and after 15 min of heat exposure (35 °C) followed by 70 min recovery at 20 °C. Scale bar, 50 μm. Right. Corresponding quantification of the fluorescence level per animal. Scatter dot plot with relative expression of Phsp16.2::GFP (normalized to wild type of each independent experiment) (line shows median). n = 10–20 animals per condition distributed across three to four independent experiments. One-way ANOVA and Dunn’s post hoc test for multiple comparisons among groups in basal conditions were used. ns, not significant. One-way ANOVA and Dunn’s post hoc test for multiple comparisons among groups after heat stress were used **p p t test (basal vs. heat stress) was used. ****p (B) Log2 fold-changes in hsp-16.2 transcript levels in animals exposed 15 min of heat exposure (35 °C). Negative and positive values indicate down- and up-regulation of this gene compared to wild-type animals, respectively. Fold change was calculated as ΔCt basal conditions/ΔCt test conditions. Results are shown as mean ± s.e.m. The data underlying this figure can be found at https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s004
(JPG)
S5 Fig. HSF-1 is a minor player in the modulation of the stress response by INS-3.
(A) Survival percentages of wild-type animals, ins-3 single mutants, hsf-1 single mutants and ins-3;hsf-1 double mutants exposed to oxidative (left) and heat stress (right). Results are shown as mean ± s.e.m. Five and six independent experiments were performed for oxidative stress and heat, respectively. Each experiment included 50–100 worms per condition. (B) Oxidation resistance of animals subjected to intestinal RNAi-mediated silencing of hsf-1. The VP303 (kbIs7 [nhx-2p::rde-1 + rol-6(su1006)]) background allows RNAi silencing only in the intestine [55,109,110]. RNAi, RNA interference. Results are shown as mean ± s.e.m. Results are shown as mean ± s.e.m. Four independent experiments were performed (n = 4). Each experiment included 30–80 worms per condition. The data underlying this figure can be found at https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s005
(JPG)
S6 Fig.
ins-4 expression is enhanced upon oxidative stress.
Left. Representative epifluorescence images (20×) of young non-gravid adults expressing Pins-4::GFP under basal conditions, oxidative (2 h, 1 mM FeSO4) or heat stress (6 h, 30 °C). Scale bar, 50 µm. Right. Corresponding quantification of fluorescence levels per worm. Scatter dot plot with relative expression of Pins-4::GFP normalized to the basal condition of each independent experiment. Line at the median. n = 45–60 animals per condition distributed across (three) independent experiments. One-way ANOVA (Kruskal–Wallis test) and Dunn’s post hoc test versus basal were used. ns, not significant, ***p https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s006
(JPG)
S7 Fig. Tyramine signaling mutants have no obvious defects in the defecation motor program.
(A) Representative kymographs of intestinal GCaMP fluorescence in freely behaving animals on NGM plates seeded with OP50. The anterior or the worm is oriented toward the top of each kymograph. The mean fluorescent intensity (white trace) is superimposed on the kymograph with a y-axis range of −1,000–8,000 arbitrary units (a.u.). Colormap values are indicated on the right. (B) Quantification of defecation interval during 5-min recordings in wild-type (n = 7 animals), tdc-1 (n = 6 animals), and tyra-3 (n = 10 animals) mutants. Defecation events occur every 45−50 s in wild-type, tdc-1, and tyra-3 mutant animals. Results are mean ± s.e.m, one-way ANOVA with Dunnett’s multiple comparison test. ns, not significant. The data underlying this figure can be found at https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s007
(JPG)
S8 Fig. Exogenous tyramine alters intestinal Ca2+ waves in tyramine receptor quadruple mutants.
Measurement of intestinal GCaMP fluorescence in lgc-55; ser-2, tyra-2, tyra-3 quadruple mutants exposed to 30 mM tyramine. Kymographs are oriented with the anterior of the animal at the top. The mean fluorescent intensity (white trace) is superimposed on the kymograph with a y-axis range of −1,000–8,000 arbitrary units (a.u.). Colormap values for the kymograph are indicated on the right. Twenty-two animals were recorded in total. The data underlying this figure can be found at https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s008
(JPG)
S9 Fig. INS-3 is tonically released from the intestine under basal conditions.
Representative image (40×) of L4-staged worm carrying Pges-1::INS-3::VENUS in basal conditions showed on differential interference contrast (DIC), fluorescence, and merged. Note the fluorescence both in the intestine (faint) and in the coelomocytes. Scale bar, 50 μm.
https://doi.org/10.1371/journal.pbio.3002997.s009
(JPG)
S10 Fig. Exogenous tyramine does not up-regulate the transcription of Pges-1::INS-3::VENUS.
Log2 fold-changes in Venus transcript levels in transgenic animals expressing Pges-1::ins-3::venus exposed to exogenous tyramine (15 mM). Negative and positive values indicate down- and up-regulation of the transcript compared to non-exposed to non-exposed animals. Fold change was calculated as ΔCt no Tyr/ΔCt Tyr. Results are shown as mean ± s.e.m. The data underlying this figure can be found at https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s010
(JPG)
S11 Fig. Tyraminergic modulation of stress resistance through intestinal INS-3 release requires the G-protein-coupled receptor TYRA-3.
Survival percentages to oxidative (left) and heat stress (right) of wild-type, tyra-3 null mutants, and animals expressing the intestinal rescue of ins-3 on wild-type and tyra-3 null mutant backgrounds in the absence or presence of exogenous tyramine (15 mM), (mean ± s.e.m). Four independent experiments were performed (n = 4). Each experiment included 40−80 animals per condition. For conditions with tyramine, a two-tailed Student’s t test versus the same strain without tyramine was used. ns, not significant, * p t test was used between tyra-3 null mutants and tyra-3; ins-3; Pelt-2::INS-3 strain. ns, not significant. The data underlying this figure can be found at https://osf.io/wfgvs/.
https://doi.org/10.1371/journal.pbio.3002997.s011
(JPG)
S1 Video. Rhythmic Ca2+ waves propagate from the posterior to the anterior end of the intestine in wild-type animals every 45–50 s.
Representative video showing intestinal calcium transients in a wild-type animal expressing GCaMP6 in the intestine in the presence of food.
https://doi.org/10.1371/journal.pbio.3002997.s012
(MP4)
S2 Video. Exogenous tyramine (30 mM) triggers a dramatic increase in Ca2+ transients in the intestine.
Representative video showing intestinal calcium transients generated by 30 mM tyramine in a wild-type animal expressing GCaMP6 in the intestine in the presence of food.
https://doi.org/10.1371/journal.pbio.3002997.s013
(MP4)
S3 Video. Exogenous tyramine (30 mM) triggers high-frequency Ca2+ transients even in the absence of food.
Representative video showing intestinal calcium transients generated by 30 mM tyramine in a wild-type animal expressing GCaMP6 in the intestine in the absence of food.
https://doi.org/10.1371/journal.pbio.3002997.s014
(MP4)
S4 Video. Tyramine-triggered intestinal Ca2+ transients are significantly reduced in tyra-3 null mutants.
Representative video showing intestinal calcium transients generated by 30 mM tyramine in a tyra-3 null mutant animal expressing GCaMP6 in the intestine.
https://doi.org/10.1371/journal.pbio.3002997.s015
(MP4)
S5 Video. Tyramine-triggered intestinal Ca2+ transients are mostly abolished in itr-1 null mutants.
Representative video showing intestinal calcium transients generated by 30 mM tyramine in an itr-1 null mutant animal expressing GCaMP6 in the intestine.
https://doi.org/10.1371/journal.pbio.3002997.s016
(MP4)
S1 Table.
ins-3 is expressed in ins-3 null mutants rescued with the Prgef-1::ins-3 transgene.
ΔCT values of ins-3 measured by qPCR in ins-3 null mutants and animals rescued with the Prgef-1::ins-3 transgene. These data support effective neuronal expression of ins-3 in the rescued animals.
https://doi.org/10.1371/journal.pbio.3002997.s017
(PDF)
Acknowledgments
Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We thank Andrés Garelli for the helpful discussions. In addition, we would like to acknowledge Ignacio Bergé, Andrea Thackeray, Adrian Bizet, Carolina Gomila, Marta Stulhdreher, and Carla Chrestía for technical support. We thank Marian Walhout for strains.
References
- 1.
Rossnerova A, Izzotti A, Pulliero A, Bast A. The molecular mechanisms of adaptive response related to environmental stress. Int J Mol Sci. 2020;21(19). pmid:32992730 - 2.
Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: universality and adaptive individual differences. Dev Rev. 2006;26(2):175–212. - 3.
Nussey DH, Wilson AJ, Brommer JE. The evolutionary ecology of individual phenotypic plasticity in wild populations. J Evol Bio. 2007;20(3):831–44. Epub 2007/05/01. pmid:17465894 - 4.
Auer SK, Salin K, Rudolf AM, Anderson GJ, Metcalfe NB. Flexibility in metabolic rate confers a growth advantage under changing food availability. J Anim Ecol. 2015;84(5):1405–11. Epub 2015/05/06. pmid:25939669; PMCID: PMC4682473 - 5.
Zhang N, Cao L. Starvation signals in yeast are integrated to coordinate metabolic reprogramming and stress response to ensure longevity. Curr Genet. 2017;63(5):839–43. Epub 2017/04/27. pmid:28444510; PMCID: PMC5605593 - 6.
Moreau K, Luo S, Rubinsztein DC. Cytoprotective roles for autophagy. Curr opin cell bio. 2010;22(2):206–11. Epub 2010/01/05. pmid:20045304; PMCID: PMC2860226 - 7.
Murphy CT, Hu PJ. Insulin/insulin-like growth factor signaling in C. elegans. WormBook. 2013:1–43. pmid:24395814 - 8.
Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–6. Epub 2001/04/09. pmid:11292874 - 9.
Bohni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999;97(7):865–75. Epub 1999/07/10. pmid:10399915 - 10.
Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Developmental biology. 2001;229(1):141–62. Epub 2001/01/03. pmid:11133160 - 11.
Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–7. Epub 2002/12/17. pmid:12483226 - 12.
Biglou SG, Bendena WG, Chin-Sang I. An overview of the insulin signaling pathway in model organisms Drosophila melanogaster and Caenorhabditis elegans. Peptides. 2021;145:170640. Epub 2021/08/28. pmid:34450203 - 13.
Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–12. pmid:20336132 - 14.
Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92(16):7540–4. pmid:7638227 - 15.
Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13(11):1385–93. pmid:10428762 - 16.
Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–4. Epub 2003/01/25. pmid:12543978 - 17.
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–4. pmid:8247153 - 18.
Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49(6):B270–6. pmid:7963273 - 19.
Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, et al. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes & Development Genes Dev; 2001. p. 672–86. - 20.
Chen Z, Hendricks M, Cornils A, Maier W, Alcedo J, Zhang Y. Two insulin-like peptides antagonistically regulate aversive olfactory learning in C. elegans. Neuron. 2013;77(3):572–85. Epub 2013/02/12. pmid:23395381; PMCID: PMC3569836 - 21.
Matsunaga Y, Nakajima K, Gengyo-Ando K, Mitani S, Iwasaki T, Kawano T. A Caenorhabditis elegans insulin-like peptide, INS-17: its physiological function and expression pattern. Biosci Biotechnol Biochem. 2012;76(11):2168–72. pmid:23132577 - 22.
Matsunaga Y, Honda Y, Honda S, Iwasaki T, Qadota H, Benian GM, et al. Diapause is associated with a change in the polarity of secretion of insulin-like peptides. Nat Commun. 2016;710573. pmid:26838180 - 23.
Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137(4):671–80. Epub 2010/01/30. pmid:20110332; PMCID: PMC2827619 - 24.
Ritter AD, Shen Y, Fuxman Bass J, Jeyaraj S, Deplancke B, Mukhopadhyay A, et al. Complex expression dynamics and robustness in C. elegans insulin networks. Genome Res. 2013;23(6):954–65. pmid:23539137 - 25.
Zheng S, Chiu H, Boudreau J, Papanicolaou T, Bendena W, Chin-Sang I. A functional study of all 40 Caenorhabditis elegans insulin-like peptides. J Biol Chem. 2018;293(43):16912–22. pmid:30206121; PMCID: PMC6204898. - 26.
Kao G, Nordenson C, Still M, Rönnlund A, Tuck S, Naredi P. ASNA-1 positively regulates insulin secretion in C. elegans and mammalian cells. Cell. 2007;128(3):577–87. pmid:17289575 - 27.
Yoshina S, Mitani S. Loss of C. elegans GON-1, an ADAMTS9 homolog, decreases secretion resulting in altered lifespan and dauer formation. PLoS One. 2015;10(7):e0133966. Epub 2015/07/29. pmid:26218657; PMCID: PMC4517882 - 28.
Mesa R, Luo S, Hoover CM, Miller K, Minniti A, Inestrosa N, et al. HID-1, a new component of the peptidergic signaling pathway. Genetics. 2011;187(2):467–83. pmid:21115972; PMCID: PMC3030490 - 29.
Arun CP. Fight or flight, forbearance and fortitude: the spectrum of actions of the catecholamines and their cousins. Ann N Y Acad Sci. 2004;1018:137–40. Epub 2004/07/09. pmid:15240362. - 30.
McCarty R. Chapter 4 – The fight-or-flight response: a cornerstone of stress research. In: Fink G, editor. Stress: concepts, cognition, emotion, and behavior. San Diego: Academic Press; 2016. p. 33–7. - 31.
Mronz M, Lehmann FO. The free-flight response of Drosophila to motion of the visual environment. J Exp Biol. 2008;211(Pt 13):2026–45. Epub 2008/06/17. pmid:18552291 - 32.
De Rosa MJ, Veuthey T, Florman J, Grant J, Blanco MG, Andersen N, et al. The flight response impairs cytoprotective mechanisms by activating the insulin pathway. Nature. 2019;573(7772):135–8. pmid:31462774 - 33.
Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46(2):247–60. pmid:15848803 - 34.
Donnelly JL, Clark CM, Leifer AM, Pirri JK, Haburcak M, Francis MM, et al. Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 2013;11(4):e1001529. pmid:23565061 - 35.
Maguire SM, Clark CM, Nunnari J, Pirri JK, Alkema MJ. The C. elegans touch response facilitates escape from predacious fungi. Curr Bio. 2011;21(15):1326–30. Epub 2011/08/02. pmid:21802299; PMCID: PMC3266163 - 36.
Pirri JK, McPherson AD, Donnelly JL, Francis MM, Alkema MJ. A tyramine-gated chloride channel coordinates distinct motor programs of a Caenorhabditis elegans escape response. Neuron. 2009;62(4):526–38. pmid:19477154 - 37.
Pirri JK, Rayes D, Alkema MJ. A change in the ion selectivity of ligand-gated ion channels provides a mechanism to switch behavior. PLoS Biol. 2015;13(9):e1002238. pmid:26348462 - 38.
Zhang YP, Zhang WH, Zhang P, Li Q, Sun Y, Wang JW, et al. Intestine-specific removal of DAF-2 nearly doubles lifespan in Caenorhabditis elegans with little fitness cost. Nat Commun. 2022;13(1):6339. Epub 2022/10/26. pmid:36284093; PMCID: PMC9596710 - 39.
Senchuk MM, Dues DJ, Schaar CE, Johnson BK, Madaj ZB, Bowman MJ. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genet. 2018;14(3):e1007268. pmid:29522556. - 40.
Deng J, Dai Y, Tang H, Pang S. SKN-1 Is a Negative Regulator of DAF-16 and Somatic Stress Resistance in Caenorhabditis elegans. G3 (Bethesda). 2020;10(5):1707–12. pmid:32161088 - 41.
Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev. 2002;123(12):1579–87. pmid:12470895 - 42.
Lin XX, Sen I, Janssens GE, Zhou X, Fonslow BR, Edgar D, et al. DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and longevity. Nat Commun. 2018;9(1):4400. Epub 2018/10/26. pmid:30353013; PMCID: PMC6199276 - 43.
Chiang W-C, Ching T-T, Lee HC, Mousigian C, Hsu A-L. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148(1–2):322–34. pmid:22265419 - 44.
Hartwig K, Heidler T, Moch J, Daniel H, Wenzel U. Feeding a ROS-generator to Caenorhabditis elegans leads to increased expression of small heat shock protein HSP-16.2 and hormesis. Genes Nutr. 2009;4(1):59–67. Epub 2009/03/03. pmid:19252938; PMCID: PMC2654055. - 45.
Morton EA, Lamitina T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 2013;12(1):112–20. Epub 2012/10/31. pmid:23107491; PMCID: PMC3552056 - 46.
Link CD, Cypser JR, Johnson CJ, Johnson TE. Direct observation of stress response in Caenorhabditis elegans using a reporter transgene. Cell Stress Chaperones. 1999;4(4):235–42. Epub 1999/12/11. pmid:10590837; PMCID: PMC312938 - 47.
Kovacs D, Biro JB, Ahmed S, Kovacs M, Sigmond T, Hotzi B, et al. Age-dependent heat shock hormesis to HSF-1 deficiency suggests a compensatory mechanism mediated by the unfolded protein response and innate immunity in young Caenorhabditis elegans. Aging Cell. 2024;23(10):e14246. Epub 2024/06/19. pmid:38895933; PMCID: PMC11464127 - 48.
Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320(5877):811–4. pmid:18467592 - 49.
Finger F, Ottens F, Hoppe T. The argonaute proteins ALG-1 and ALG-2 are linked to stress resistance and proteostasis. MicroPubl Biol. 2021;2021. Epub 2021/11/02. pmid:34723149; PMCID: PMC8553546 - 50.
Lee SH, Omi S, Thakur N, Taffoni C, Belougne J, Engelmann I, et al. Modulatory upregulation of an insulin peptide gene by different pathogens in C. elegans. Virulunce. 2018;9(1):648–58. pmid:29405821 - 51.
Gilon P, Henquin JC. Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev. 2001;22(5):565–604. pmid:11588141 - 52.
Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53. pmid:22974359; PMCID: PMC3934755 - 53.
Wollheim CB, Biden TJ. Second messenger function of inositol 1,4,5-trisphosphate. Early changes in inositol phosphates, cytosolic Ca2+, and insulin release in carbamylcholine-stimulated RINm5F cells. J Biol Chem. 1986;261(18):8314–9. pmid:3522567 - 54.
Bach AG, Wolgast S, Muhlbauer E, Peschke E. Melatonin stimulates inositol-1,4,5-trisphosphate and Ca2+ release from INS1 insulinoma cells. J Pineal Res. 2005;39(3):316–23. pmid:16150114. - 55.
Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J Gen Physiol. 2005;126(4):379–92. pmid:16186564 - 56.
Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98(6):757–67. Epub 1999/09/28. pmid:10499793 - 57.
Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124(4):855–72. Epub 1990/04/01. pmid:2323555; PMCID: PMC1203977 - 58.
Florman JT, Alkema MJ. Co-transmission of neuropeptides and monoamines choreograph the C. elegans escape response. PLoS Genet. 2022;18(3):e1010091. pmid:35239681 - 59.
Mahoney TR, Luo S, Round EK, Brauner M, Gottschalk A, Thomas JH, et al. Intestinal signaling to GABAergic neurons regulates a rhythmic behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008;105(42):16350–5. pmid:18852466; PMCID: PMC2570992 - 60.
Baumeister R, Schaffitzel E, Hertweck M. Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J Endocrinol. 2006;190(2):191–202. Epub 2006/08/11. pmid:16899554 - 61.
Scott BA, Avidan MS, Crowder CM. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002;296(5577):2388–91. pmid:12065745 - 62.
Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001;15(3):627–34. pmid:11259381 - 63.
Vanfleteren JR. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J. 1993;292(Pt 2):605–8. Epub 1993/06/01. pmid:8389142; PMCID: PMC1134253 - 64.
Li C, Kim K. Neuropeptides. WormBook. 2008:1–36. Epub 2008/09/27. pmid:18819171; PMCID: PMC2749236 - 65.
Ewe CK, Alok G, Rothman JH. Stressful development: integrating endoderm development, stress, and longevity. Dev Biol. 2021;47134–48. pmid:33307045 - 66.
Uno M, Tani Y, Nono M, Okabe E, Kishimoto S, Takahashi C, et al. Neuronal DAF-16-to-intestinal DAF-16 communication underlies organismal lifespan extension in C. elegans. iScience. 2021;24(7):102706. Epub 2021/07/09. pmid:34235410; PMCID: PMC8246587 - 67.
Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402(6763):804–9. pmid:10617200 - 68.
Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290(5489):147–50. Epub 2000/10/06. pmid:11021802 - 69.
Iser WB, Gami MS, Wolkow CA. Insulin signaling in Caenorhabditis elegans regulates both endocrine-like and cell-autonomous outputs. Dev Bio. 2007;303(2):434–47. Epub 2007/01/30. pmid:17257591; PMCID: PMC2262836 - 70.
Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100(9):5034–9. Epub 2003/04/19. pmid:12702762; PMCID: PMC154293 - 71.
Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44(4):406–12, S1. Epub 2012/03/13. pmid:22406641; PMCID: PMC3315609 - 72.
Srivastava S, Pandey H, Singh SK, Tripathi YB. GLP 1 Regulated intestinal cell’s insulin expression and selfadaptation before the onset of type 2 diabetes. Adv Pharm Bull. 2019;9(2):325–30. Epub 2019/08/06. pmid:31380261; PMCID: PMC6664108 - 73.
Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, et al. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27(49):13402–12. pmid:18057198 - 74.
Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56(4):1087–94. Epub 2007/03/31. pmid:17395749; PMCID: PMC1853382 - 75.
Sassmann A, Gier B, Grone HJ, Drews G, Offermanns S, Wettschureck N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Investig. 2010;120(6):2184–93. Epub 2010/05/05. pmid:20440069; PMCID: PMC2877950 - 76.
Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006;27(6):621–76. pmid:16868246 - 77.
Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell. 2007;6(5):715–21. pmid:17711560 - 78.
Swanson MM, Riddle DL. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev Biol. 1981;84(1):27–40. pmid:7250500 - 79.
Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9(3):399–431. - 80.
Selye H. Stress without distress: Philadelphia. New York: J.B. Lippincott; 1974. - 81.
Boonstra R. Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol. 2013;27:13. - 82.
Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24(2):91–8. Epub 2001/02/13. pmid:11164939 - 83.
Odio M, Brodish A, Ricardo MJ Jr. Effects on immune responses by chronic stress are modulated by aging. Brain, behav Immun. 1987;1(3):204–15. Epub 1987/09/01. pmid:3509811 - 84.
Travers M, Clinchy M, Zanette L, Boonstra R, Williams TD. Indirect predator effects on clutch size and the cost of egg production. Ecol Lett. 2010;13(8):980–8. pmid:20528899. - 85.
Aamodt EJ, Chung MA, McGhee JD. Spatial control of gut-specific gene expression during Caenorhabditis elegans development. Science. 1991;252(5005):579–82. pmid:2020855 - 86.
Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2-3):193–210. pmid:24798553 - 87.
Glaser R, Kiecolt-Glaser J. How stress damages immune system and health. Discov Med. 2005;5(26):165–9. pmid:20704904. - 88.
Groer MW, Kane B, Williams SN, Duffy A. Relationship of PTSD symptoms with combat exposure, stress, and inflammation in American soldiers. Biol Res Nurs. 2015;17(3):303–10. pmid:25202037 - 89.
Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol Psychiatry. 2014;19(11):1156–62. Epub 2014/09/24. pmid:25245500; PMCID: PMC4211971 - 90.
Porhomayon J, Kolesnikov S, Nader ND. The impact of stress hormones on post-traumatic stress disorders symptoms and memory in cardiac surgery patients. J Cardiovasc Thorac Res. 2014;6(2):79–84. pmid:25031821; PMCID: PMC4097856 - 91.
Modan M, Halkin H. Hyperinsulinemia or increased sympathetic drive as links for obesity and hypertension. Diabetes Care. 1991;14(6):470–87. pmid:1864220 - 92.
Mellon SH, Bersani FS, Lindqvist D, Hammamieh R, Donohue D, Dean K. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS One. 2019;14(3):e0213839. pmid:30883584 - 93.
Aaseth J, Roer GE, Lien L, Bjørklund G. Is there a relationship between PTSD and complicated obesity? A review of the literature. Biomed Pharmacother. 2019;117108834. pmid:31177066 - 94.
Michopoulos V, Vester A, Neigh G. Posttraumatic stress disorder: A metabolic disorder in disguise? Experimental neurology. 2016;284(Pt B):220–9. Epub 2016/06/02. pmid:27246996; PMCID: PMC5056806 - 95.
Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. pmid:4366476; PMCID: PMC1213120 - 96.
Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. pmid:18050451 - 97.
Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154(5):1023–35. pmid:23972393 - 98.
Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Bio. 2001;11(24):1975–80. Epub 2001/12/19. pmid:11747825. - 99.
Kenyon C. The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366(1561):9–16. pmid:21115525 - 100.
Kwon ES, Narasimhan SD, Yen K, Tissenbaum HA. A new DAF-16 isoform regulates longevity. Nature. 2010;466(7305):498–502. Epub 2010/07/09. pmid:20613724; PMCID: PMC3109862. - 101.
Conte D Jr, MacNeil LT, Walhout AJM, Mello CC. RNA Interference in Caenorhabditis elegans. Curr Protoc Mol Biol. 2015;109:26–30. pmid:25559107 - 102.
Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421(6920):231–7. Epub 2003/01/17. pmid:12529635 - 103.
Andersen N, Veuthey T, Blanco MG, Silbestri GF, Rayes D, De Rosa MJ. 1-Mesityl-3-(3-Sulfonatopropyl) imidazolium protects against oxidative stress and delays proteotoxicity in C. elegans. Front Pharmacol. 2022;13:908696. pmid:35685626 - 104.
Edelstein AD, Tsuchida MA, Amodaj N, Pinkard H, Vale RD, Stuurman N. Advanced methods of microscope control using muManager software. J Biol Methods. 2014;1(2). pmid:25606571; PMCID: PMC4297649 - 105.
Hoover CM, Edwards SL, Yu SC, Kittelmann M, Richmond JE, Eimer S, et al. A novel CaM kinase II pathway controls the location of neuropeptide release from Caenorhabditis elegans motor neurons. Genetics. 2014;196(3):745–65. Epub 2014/03/22. pmid:24653209; PMCID: PMC3948804. - 106.
Sieburth D, Madison JM, Kaplan JM. PKC-1 regulates secretion of neuropeptides. Nat Neurosci. 2007;10(1):49–57. Epub 2006/11/28. pmid:17128266 - 107.
Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20(1):87–90. Epub 2001/12/26. pmid:11753368 - 108.
Fay DS. Classical genetic methods. WormBook. 2013:1–58. pmid:24395816; PMCID: PMC4127492 - 109.
Wu C, Karakuzu O, Garsin DA. Tribbles pseudokinase NIPI-3 regulates intestinal immunity in Caenorhabditis elegans by controlling SKN-1/Nrf activity. Cell Rep. 2021;36(7):109529. Epub 2021/08/19. pmid:34407394; PMCID: PMC8393239 - 110.
Zečić A, Braeckman BP. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells. 2020;9(1):109.Epub 2020/01/08. pmid:31906434; PMCID: PMC7017163
ADVERTISEMENT:
Hello, para pencinta slot! pernahkah mendengar semboyan “raja slot? Kalau tidak, bersiaplah jatuh hati dengan program ini. raja slot merupakan mesin slots yang sering kasih kemenangan. Yup, mesin-mesin ini bisa dikatakan adalah jagoannya buat membawa come back hasil. tapi, cemana sih
tekniknya nemuin slot gaco yang tepat? Santuy Bro, kita bahas tenang saja di sini
Permainan tergacor saat ini satu-satunya berada Indonesia yaitu akan memberikan ROI tertinggi
SEGERA hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia