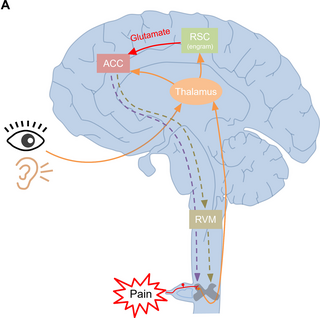
Accumulative evidence supports that the ACC is crucial for pain processing and pain-related emotional regulation [2,3,18]. The activity of excitatory neurons, synaptic transmission, and plasticity in the ACC are thought to be the underlying mechanisms of pain processing and its related emotional disorders [3]. In recent years, the exploration of interactions between the ACC and various other brain regions remains a central focus in the field of pain and pain-related research. However, there is still a lack of detailed electrophysiological and behavioral relevant data and understanding in the regulatory network of pain and pain-related emotions. In this study, we identified a new cortico-cortical pathway that contributes to acute mechanical and thermal nociceptive perception in mice. The ACC received the direct excitatory projections from the RSC. This excitatory synaptic transmission is mainly mediated by postsynaptic AMPA receptors and coupled with the elevation of the intracellular Ca2+ level in the ACC. Phenotypically, activating the RSC-ACC pathway facilitates both mechanical and thermal nociceptive perception at a supraspinal level without affecting spinal nociceptive transmission directly. Nevertheless, this pathway did not participate in the regulation of anxiety-like and aversive behaviors. It is the first report to reveal the specific nociceptive facilitation mediated by the RSC-ACC pathway. This finding suggests that the processing of pain and pain-related emotional information in the brain may be regioselective and separated among different cortical areas.
The ACC-mediated network for pain and emotion regulation
As a hub for processing sensory, emotional, and cognitive information, ACC integrates plenty of neural inputs from various brain regions, including sensory, limbic, and cortical brain regions, etc., distinctly contributing to pain and pain-related emotions [3,35]. Recent research has demonstrated robust connections from the thalamus to the ACC in mice. Specifically, the ACC receives extensive projections from multiple thalamic subnuclei, including the anterior, ventral, medial, and lateral thalamic nuclei [36]. Electrophysiological investigations have revealed that stimulation of the thalamus induces short-term plastic changes in the ACC, facilitating the transmission of nociceptive information [4,37,38]. Moreover, the inputs from the mediodorsal thalamus (MD) to the ACC have been reported to elicit chronic pain-related aversion [39]. Besides the main inputs from the thalamus, the amygdala can relay the ascending nociceptive information to the ACC for pain and pain-related emotion regulation [3,39,40]. The pathway from the basolateral nucleus of the amygdala (BLA) to the ACC is crucial for the regulation of chronic pain and depression comorbidity [40]. Another study proved that activation of the ascending locus coeruleus(LC)-ACC noradrenergic projections facilitated pain and itch responses by enhancing glutamatergic synaptic transmission and neural excitability within the ACC [41]. Conversely, the GABAergic projections from the PVN and medial prefrontal cortex (mPFC) to ACC are found to attenuate neuropathic pain as well as comorbid anxiety behaviors by different types of interneurons [7,22]. In our research, we identified a novel cortico-cortical neural pathway that functions in the mediation of pain perception, contributing to a better understanding of the pain regulatory network within the brain. We observed that bilateral neurons in the ACC receive direct excitatory input from the unilateral RSC. Furthermore, optogenetic stimulation of the unilateral RSC-ACC projections resulted in a decrease in the bilateral mechanical thresholds of mouse hindpaws. According to another study, the ACC-ACC excitatory connections (from ipsilateral to contralateral side crossing the corpus callosum) contribute to bilateral pain perception. These findings may explain the reason why unilateral activation of the RSC-ACC pathway induces bilateral allodynia of mice in our behavioral tests.
Synaptic characteristics in cortico-cortical connections
Although many anatomic studies reveal the projections from different cortical and subcortical areas to the ACC [31,32,36]. Few studies have provided direct evidence to describe the characteristics of synaptic transmission of these projections. In the present study, we utilize electrophysiological techniques and optogenetics to explore the features of the RSC-ACC projection in vitro. Through sagittal brain slice preparations, we successfully recorded eEPSCs via electrical stimulation and optogenetics within the RSC. Further investigations confirmed that these synaptic connections were monosynaptic. Pharmacological assays using different glutamate receptor antagonists demonstrated that the eEPSCs in the ACC are primarily mediated by AMPA receptors without any component of KA receptors. This finding contrasts with typical scenarios where the part of residual eEPSCs, induced by in situ, contralateral, or thalamic stimulation, were mediated by KA receptors [34,42]. Wu and colleagues identified these residual currents and established their mediation through GluK1 and GluK2 receptors in specific KA receptor knockout mice [34]. This indicates a heterogeneity of cortical synapses within the ACC. Distinct synaptic connections are responsible for distinct functions. We propose at least 3 different types of excitatory synapses: silent synapses; AMPA receptor-containing synapses; and mixed synapses comprising both AMPA and KA receptors. Although direct evidence for silent synapses in the ACC remains elusive [43], our previous electrophysiological data consistently suggested not only their existence or pure NMDA receptor-mediated responses but also their recruitment through long-term potentiation (LTP) or chemical induction [44–46]. Our findings provide new evidence supporting pure AMPA receptor-containing excitatory synapses, similar to well-characterized excitatory synapses found in the CA1 region of the hippocampus [47]. However, it is unclear if such AMPA synapses are common in other cortico-cortical connections. Lastly, we also observed that the projections from side-to-side ACC contain mixed-type synapses expressing both AMPA and KA receptors [48]. Comprehensive studies are warranted to delineate the characteristics of these diverse synapse types throughout the entire brain rather than focusing solely on the ACC.
The modulation of intracellular Ca2+ signal in the ACC
It is well established that intracellular Ca2+ and Ca2+ channels play pivotal roles in neural excitability and function, particularly in neurotransmitter release and synaptic transmission [49,50]. In neurons, cytosolic Ca2+ influences various downstream signaling pathways and gene expression to regulate cellular physiology and development. For example, elevated cytosolic Ca2+ concentrations can activate classical PKA/PKC/CaMKII signaling cascades, leading to cAMP-response element binding protein (CREB) activation and promoting protein synthesis-dependent synaptic plasticity such as LTP [3,51–53]. Our previous study provided electrophysiological evidence indicating that N-type voltage-gated calcium channels (VGCCs) primarily mediate fast synaptic transmission within ACC [54]. Regarding postsynaptic Ca2+ dynamics, Li and colleagues reported that both NMDA receptors and VGCC-mediated action potentials induce significant Ca2+ influx in ACC pyramidal neurons. This influx was also observed during the induction phase of LTP, emphasizing the close relationship between intracellular Ca2+ signaling pathways and synaptic plasticity [33]. Utilizing in vivo Ca2+ imaging techniques on awake mice, a recent study demonstrated that increased amplitude of the Ca2+ signal in layer 5 pyramidal neurons of the ACC correlates with mechanical stimulation intensity. A single noxious pain stimulus elicited substantial Ca2+ transients; conversely, non-noxious stimuli had negligible effects in the ACC [55]. Collectively, these findings suggest that Ca2+ channels along with their associated signaling pathways significantly contribute to pain processing in ACC neurons. In this study, we discovered that activation of the RSC-ACC projections markedly elevates cytosolic Ca2+ levels in ACC pyramidal neurons—potentially influencing changes in synaptic plasticity such as LTP or silent synapse recruitment. We are continuing our investigation into how these calcium signaling pathways regulate the RSC-ACC connectivity.
Functional implications of the RSC-ACC pathway
RSC has been implicated in various high-level cognitive functions, including spatial navigation, associative learning, and memory formation [25,26,56,57]. Numerous prior studies have indicated a close relationship between the RSC and nociceptive perception [27,28,58,59]. Electrophysiological recordings demonstrated enhanced responses of RSC neurons to cutaneous noxious stimulation in rabbits [58]. While most research involving both humans and animals suggests that the ACC and insular cortex (IC) play critical roles in pain perception and chronic pain management, it is inferred that the role of the RSC may be preferentially modulatory rather than central [6,9,60]. However, our findings provide direct and compelling evidence that excitatory projections from the RSC to ACC are also significant for nociceptive processing under physiological conditions. Activation of the RSC-ACC pathway facilitated behavioral responses to both noxious mechanical and thermal stimuli in naturally behaving mice. It has been reported that there are two descending pathways contributing to nociceptive facilitation: via direct cortical-spinal projections [61] or brainstem relay [62–64]. However, this conflicts with our behavioral results. According to our behavioral results, activation of the RSC-ACC pathway did not influence response latency in the spinal tail-flick test. We consider that the RSC-ACC connection modulates perceptions of both mechanical and thermal nociception at a supraspinal level. This modulation occurs through the direct excitation of ACC neurons without affecting descending facilitatory mechanisms to amplify the nociceptive inputs. Our results align with the theory that forebrain regions are essential for integrating sensory inputs with motor responses, which is necessary for withdrawal behaviors in the hot-plate test [65]. In our hot plate tests, we found that activating all the RSC-ACC projecting neurons by hSyn-ChR2 only facilitated the withdrawal responses to 50°C, but not to 55°C thermal stimuli. However, specifically activating the excitatory projecting neurons by CaMKII-hM3Dq significantly decreased the response latency in the 55°C hot plate test. Besides the methodological differences, we think that hSyn promoter may activate other potential regulatory components, such as interneurons or neuropeptides, probably leading to the differences of thermal sensitization which could be only detected in 50°C hot plate test. The roles of the other neural components in the RSC-ACC pathway are required to be further explored. Our work thus offers a more comprehensive understanding of regulatory networks involved in pain processes—particularly among different cortices. Consequently, we propose a novel mechanism whereby cognitive or non-sensory responses interact with nociceptive perception without disrupting spinal sensory transmission or reflexive actions. Additionally, it is posited that the RSC-ACC pathway is involved in modulating pain-related contextual memory due to its nociceptive facilitation under physiological conditions.
In summary, we have identified a novel direct nociceptive pathway connecting the RSC to the ACC. The activation of this pathway can facilitate supraspinal nociceptive processing. Notably, our findings confirm the heterogeneity of cortical glutamatergic synapses in the brain. This heterogeneity undoubtedly contributes to the complexity of cortical functions. Furthermore, our results elucidate a new mechanism for the selective enhancement of supraspinal pain processing, which occurs without influencing spinal reflexive responses.
Experimental model and subject details
Animals.
Adult (aged 6 to 8 weeks) C57BL/6 male mice were purchased from the Experimental Animal Center of Fujian Medical University. Experimental animals were housed in plastic cages with ad libitum access to enough water and mouse chow, and the holding room was kept under standard laboratory conditions (12 h/12 h day/night cycle, temperature of 22 to 25°C, air humidity of 55% to 60%). Mice were raised under standard laboratory conditions at least 1 week before all animal experiments were carried out. All experimental procedures were performed following the guidelines approved by the Ethics Committee of Fujian Medical University and Forevercheer Medicine Pharmac Inc. (Qingdao). The license number of the ethical approval for the animal experiments: IACUC FJMU 2024-Y-0586 and ECAU FMPI 2023020627.
Drug application.
All the chemicals and drugs were obtained from Tocris Cookson (Bristol, United Kingdom) and MedChemExpress (New Jersey, United States of America). Selective competitive NMDA receptor antagonist D-AP5 was prepared in distilled water. Non-competitive AMPA receptor antagonist GYKI53655 hydrochloride and selective non-NMDA ionotropic glutamate receptors antagonist CNQX were dissolved in dimethyl sulfoxide (DMSO). GABAA receptor antagonist Picrotoxin was dissolved in ethanol as a stock solution. 4-AP was dissolved in distilled water with ultrasonic assistance. All these stock solutions were diluted to the final desired concentration in the artificial cerebrospinal fluid (ACSF) before immediate use. The DMSO and ethanol diluted in ACSF did not affect basal synaptic transmission and plasticity.
Anatomy and imaging.
In the trans-monosynaptic retrograde tracing experiments, the viruses AAV2/9-hSyn-EGFP-2a-TVA-2a-RVG-WPRE-pA (200 nL, 2.0 × 1012 genomics copies/ml, brainvta) and RV-EnvA-ΔG-DsRed (2.0 × 108 genomic copies/ml, brainvta) were separately injected into the right ACC. After a 3-week expression, the mice with the full viral infection were deeply anesthetized and perfused with 0.01 M PBS, followed by 100 ml of 4% PFA in PBS (pH 7.4). The whole brain was immediately separated and stored in the 4% PFA solution for 4-h post-fixation. Then, the whole brain was placed into 0.1 M PB containing 30% (w/v) sucrose solution for 3-d dehydration at 4°C and cut into 30 μm-thickness coronal brain slices using a freezing microtome (Leica CM1900). Sections were collected in sequence and every third section was mounted onto the slides. These sections were counterstained with DAPI (ABS9235, absin, Shanghai) and observed using a laser scanning confocal microscope (FV3000, Olympus, Japan) or a slide scanner (Slideview VS200, Olympus).
For trans-monosynaptic retrograde tracing experiments, we used a fast and high-resolution VISoR imaging method as previously described [29,30]. The separated whole brain was placed into 4% acrylamide hydrogel monomer solution (w/v, HMS) in PBS for 2 days at 4°C. Next, the whole brain was embedded with equal volume mixed solution containing 4% HMS and 20% bovine serum albumin (BSA) at 37°C for 4 h and cut into 300-μm thickness coronal sections. These sections were transferred into 5% PBS-Triton clearing solution for 24 h at 37°C with gentle shaking to increase membrane permeability. After clearing, these sections were washed 3 times with PBS and mounted onto the quartz slides in sequence. The quartz slide with fixed sections was immersed into the refractive-index-matching solution and these sections were visualized with synchronized beam-scan illumination and camera-frame readout (10× objective). The resultant voxel size is 0.5 × 0.5 × 3.5 μm3.
Multichannel field potential recordings.
For extracellular field potential recordings, we performed a 64-channel recording system (MED64, Alpha-Med Sciences, Japan) throughout the experiments as previously described42. The MED64 P5001A probe contained 64 planar microelectrodes (50 × 50 μm/each) with a 150-μm interpolar distance. Before experiments, the surface of the MED64 P5001A probe was pre-treated overnight with 0.1% polyethyleneimine (Sigma Aldrich, St. Louis, MO; P-3143) in 25 mM borate buffer (pH 8.4) at room temperature to enhance surface hydrophilicity. The sagittal brain slice was prepared as mentioned above and transferred into the recording chamber after 1-h incubation. The ACC and RSC regions were covered separately onto the microelectrodes of P5001A probe and a fine mesh anchor was used to ensure slice stability during entire recordings. The slice was continuously perfused with oxygenated, fresh ACSF at 28 to 30°C and maintained at the flow rate of 2 to 3 ml/min throughout the entire experimental period. After a minimum 1-h recovery period, one channel located in the RSC was chosen as the optimum stimulus site, which can induce the best synaptic response in the ACC after a biphasic constant current pulse test stimulus (0.2 ms) was delivered. The channel with fEPSP induced by electrical stimulus was regarded as an activated channel. The fEPSP response was sampled once every minute and averaged every 2 traces.
Two-photon Ca2+ imaging.
For two-photon calcium imaging, the virus AAV2/9-hSyn-GCamp6s-WPRE-hGHpA (150 nL, 1.6 × 1012 genomics copies/ml, brainvta) was injected into the bilateral ACC and expressed for at least 7 days. Two-photon Ca2+ imaging was performed by using a Scientifica Hyperscope with a 16 × 0.8 NA water-immersion lens (CFI75 LWD, Nikon) and Coherent laser (Chameleon Ultra II, tuning range from 680 to 1,080 nm, averaged power > 3.5 W) tuned at 900 nm for two-photon excitation for GCaMP6s. During two-photon imaging, the fluorescent baseline was first recorded at least for 20 s with the scanning parameters (1 frame/s and 512 × 512 pixels). After the baseline recording, different stimuli were applied in the RSC, at least 1.2 mm away from the imaging region in the ACC. The electrical stimulations (5 Hz, 5 ms pulse, and 195 ms interval, 9 V, 10 s) were delivered by a bipolar tungsten stimulating electrode in the RSC. In the puff experiments, 1 mM glutamate was released for 10 s just above the adjacent RSC through the whole-cell recording pipettes by using the MPPI-3 pressure injector (5~10 psi). The brain slices were perfused until the fluorescence was restored to the basal level after stimulus treatments. The obtained image data was analyzed with Image J. The fluorescent signals were quantified by measuring the mean pixel intensities of the cell body of each neuron. The fluorescent change was defined as ΔF/F0 = (Ft−F0)/F0. Ft was the fluorescent intensity at time t, and F0 was the mean of the baseline intensity before the beginning of stimulus application.
In vitro whole-cell patch-clamp recordings.
Briefly, mice were anesthetized with 2% isoflurane and decapitated quickly. The whole brain was rapidly separated and transferred into ice-cold oxygenated (95% O2 and 5% CO2) cutting solution (in mM: 252 sucrose, 2.5 KCl, 6 MgSO4, 0.5 CaCl2, 25 NaHCO3, 1.2 NaH2PO4, and 10 glucose, pH 7.3 to 7.4) within a short time. The whole brain was then trimmed and glued onto the ice-cold platform of a vibrating tissue slicer (Leica VT1200S). Then, 200-μm thickness sagittal brain slices containing both the RSC and ACC regions were cut (about 4 to 5 slices) according to the Mouse Brain in Stereotaxic Coordinates (4th edition) and then transferred to a room temperature-submerged incubation chamber containing oxygenated ACSF (in mM: 124 NaCl, 2.5 KCl, 1 NaH2PO4, 1 MgSO4, 2 CaCl2, 25 NaHCO3, and 10 glucose, pH 7.3 to 7.4) for at least 1-h incubation before conducting experiments.
The whole-cell patch recordings were performed as previously described29. The recordings were performed in voltage- or current-clamp mode using a HEKA amplifier. PatchMaster and Clampfit 10.2 software were used to acquire and analyze the data. In the present study, the eEPSCs were recorded in the ACC with a HEKA amplifier, and the electrical stimulations were delivered by a bipolar tungsten stimulating electrode placed in the RSC regions. For AMPA receptor-EPSCs and action potential recordings, the recording pipettes (3 to 5 MΩ for pyramidal neurons) were filled with an internal solution containing 124 mM K-gluconate, 5 mM NaCl, 1 mM MgCl2, 0.2 mM EGTA, 2 mM MgATP, 0.1 mM Na3GTP, and 10 mM HEPES (adjusted to pH 7.2 with KOH, 290 mOsmol). Picrotoxin (100 μm) was added to block GABAA receptor-mediated inhibitory synaptic currents for EPSCs recordings in all experiments. The neurons were voltage clamped at −60 mV in the presence of D-AP5 (50 μm) for AMPA receptor-EPSCs recordings and both D-AP5 and GYKI53655 (100 μm) for KA receptor-EPSCs recordings. CNQX was added in ACSF to block selective non-NMDA ionotropic glutamate receptors. To examine synaptic responses, the I-O curves in the ACC pyramidal neurons were recorded at different stimulus intensities. To examine presynaptic functions, the PPRs were tested at different time intervals (25, 50, 75, 100, and 150 ms intervals). Action potentials were recorded in current-clamp mode by delivering stepped currents of −200 to 300 pA (400 ms duration) in increments of 20 pA. To verify the monosynaptic connections, TTX and 4-AP were added to unselectively block Na+ and K+ channels respectively for presynaptic depolarization which specifically promotes the monosynaptic glutamate release in the optogenetical experiment in vitro.
Virus injection and surgery.
Virus injection procedures were performed as previously described9. The experimental mice were anesthetized with 2% isoflurane and fixed on a stereotaxic frame to keep parallel to the reference panel. A midline incision was made in the skull and the skull was drilled on the RSC (1.70 mm posterior to the bregma, 0.20 mm lateral to the midline, 1.00 mm ventral to the skull surface) or the ACC (0.90 mm anterior to the bregma, 0.30 mm lateral to the midline, 1.40 mm ventral to the skull surface). The viruses were stereotactically pressure-injected into the target site with equal speed (40 nL/min) using a microsyringe pump (Nanoject III #3-000-207, DRUMMOND). Next, a 10-min extension was allowed for diffusion of viral particles before the microsyringe was slowly withdrawn. The experimental mice were allowed to recover for at least 2 to 3 weeks before all the experiments were performed, except that the virus RV-EnvA-ΔG-DsRed was expressed for 7 days.
Optogenetic manipulations.
For the in vitro electrophysiological experiment, the virus AAV2/9-hSyn-hChR2(H134R)-EYFP-WPRE-hGHpA (150 nL, 1.2 × 1012 genomics copies/ml, brainvta, Wuhan) was injected into the bilateral RSC and expressed for at least 2 weeks. The blue-light pulses (420~520 nm, 10~20 mW, 0.5 ms) were given by the pE-300 LED illumination system (CoolLED) for photostimulation. For the behavioral test, the virus AAV2/9-hSyn-hChR2(H134R)-EYFP-WPRE-hGHpA and AAV2/9-hSyn-EYFP-WPRE-hGHpA (150 nL, 1.2 × 1012 genomics copies/ml) was injected into the right RSC. The optic fiber cannula (length, 2 mm; 200-μm core; NA = 0.37; THINKERTECH, Nanjing) was chronically implanted into the ipsilateral ACC (0.90 mm anterior to the bregma, 0.40 mm lateral to the midline, 1.40 mm ventral to the skull surface) to activate neuronal terminals. Behavioral tests related to optogenetics were performed after a 2-week recovery. The optic fiber was connected to a fiber patch cable with a rotary joint, which was in turn connected to a fiber-coupled laser (200 mW, 465 nm, Inper Studio, Hangzhou). Mice received 465-nm blue-light illumination (5 to 20 mW, 20 Hz, 5 ms pulse) for the light-on group throughout the entire experiments in the EPM, OF tests. For von-Frey, tail-flick, and hot-plate tests, mice received blue-light illumination for 30 to 60 s before testing. Finally, all mice were sacrificed and the whole brain was sectioned to verify optic fiber implantation and viral expression. The data was excluded if the viral expression or optic fiber implantation had a deviation from the targeted regions.
Chemogenetic manipulations.
For chemogenetic experiments, virus injection surgery was performed 2 weeks before the behavioral tests. The viruses AAV2/9-hEF1a-DIO-hM3Dq-mCherry-ER2-WPRE-pA / AAV2/9-hEF1a-DIO-mCherry-ER2-WPRE-pA and AAV2/2-Retro Plus-mCaMKIIa-Cre-WPRE-pA (120 nL, 2.0 × 1012 genomic copies/ml, Taitool, Shanghai) were injected into the right RSC and ACC respectively to label the excitatory neurons in the RSC-ACC pathway. CNO (MedChemExpress, 2 mg/kg, 0.1 ml/20 g body weight) or vehicle (saline) was injected intraperitoneally at least 30 min before behavioral testing. All the behavioral experiments were finished in 2 h after the CNO or vehicle injection. CNO was dissolved in the saline with ultrasonic assistance.
Mechanical withdrawal measurement.
The mechanical hypersensitivity was determined using an up-down method with von Frey filaments (Stoelting; Wood Dale, Illinois) applied perpendicularly to the plantar surface as previously reported29. Mice were individually placed into a plastic cage with wire mesh floors and allowed to acclimate for 30 min before testing. A series of filaments (0.008, 0.02, 0.04, 0.16, 0.4, 0.6, 1, 1.4, 2.0 g) with various bending forces were applied to the plantar surface of the hindpaw until it was bent slightly and held for 3 s. Licking, biting, or sudden withdrawal of the hindpaw were defined as positive responses. An initial filament force of 0.4 g was applied to test if the mouse was sensitive to this force. If the positive response occurred, the filament force was incrementally decreased until a negative result was obtained with an interval of 3 to 5 min between 2 tests. If the mouse was insensitive to 0.4 g filament force, a stronger filament force was applied until a positive response was obtained. The hindpaw withdrawal thresholds were finally determined using the up-down method until the positive/negative responses crossed 5 times.
Hot plate test.
The mouse was placed on a hot plate set at 50 ± 1°C or 55 ± 1°C. The latency time was recorded when the reaction of the hind paw (licking, shaking, or lifting) first appeared. The cut-off times (40 s for 50°C and 20 s for 55°C) were used to avoid tissue damage. Mice were tested a total of 3 times with an inter-trial interval of 10 min. The average of 3 repeated measurements was calculated as the final latency time.
Tail-flick test.
The tail-flick reflex was measured using a 50 W projector lamp which produced noxious radiant heat. The TF latencies to reflexive removal of the tail from the heat were recorded for 3 repeated measurements with an inter-trial interval of 30 min. The cut-off time of 10 s was used to avoid heat damage to the tail.
Open field test.
The open field test was performed as previously described29. The open field consisted of an opaque cube (40 × 40 × 30.5 cm) and was divided into a center zone (20 × 20 cm) and an outer zone as the periphery. A single mouse was placed into the arena center and allowed to explore freely for 15 min with dim illumination. The movement traces were tracked using tracking master v3.0 system and all measurements (total distance, time in the center, entries) were quantified relative to the mouse body.
Elevated plus maze.
The EPM apparatus consisted of 2 open arms (30 × 5 cm) and 2 closed arms (30 × 5 × 30 cm) which were perpendicular to each other and intersected by a central platform (5 × 5 cm). The maze was 70 cm high from the floor. For each test, the mouse was individually placed into the center of the apparatus and allowed to explore freely for 5 min with dim illumination. A tracking master v3.0 system was used to track the mouse movement. The number of entries, time spent in the open arm, and total distance were quantified relative to the mouse body.
Conditioned place aversion test.
In the conditioned place aversion test (CPA), the mice were preconditioned on the first days, and they were allowed to explore the chamber for 15 min freely. The time they spent in each chamber was recorded and analyzed. On the second day, the mice with the CNO injection were paired with a randomly chosen chamber for 15 min in the morning; 4 to 6 h later, the mice with the saline treatment were paired with the other chamber for 15 min in the afternoon. After 3 days of pairing, the mice were allowed to explore all the chambers for 15 min on the fifth day. The time spent in every chamber was analyzed to understand chamber preference. The preference index was calculated as the time spent in the CNO-paired chamber minus the time spent in the saline-paired chamber.
Statistical analysis.
All data was reported as means ± SEM. OriginPro 2021, GraphPad Prism 9, and SPSS 22.0 software were used for figure plotting and data analysis. Two-tail paired or unpaired t test was used to examine statistical differences between the 2 groups. One-way ANOVA followed by Dunnett T3 post hoc test was used for comparison among multiple groups. Two-way ANOVA followed by Sidak multiple comparisons test was used to identify significant differences among multiple groups with 2 impact factors. The significance levels of the statistical tests were presented as *p p p p > 0.05.
ADVERTISEMENT:
Halo, sobat pengemar slot! Pernah mendengar semboyan “raja slot? Kalau belum, siap-siap jatuh hati sama konsep ini. slot demo adalah mesin slots yang selalu kasih kemenangan. Ya, mesin-mesin ini bisa disebut adalah jagoannya tuk bawa come back cuan. tapi, gimana sih
tekniknya nemuin slot gacor yang tepat? Santai Bro, kita bahas tenang saja di sini
Games terbaik waktu ini hanya satu di Indonesia hanya di akan memberikan imbal hasil tertinggi
Daftarkanlah hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia