Abstract
The peritrophic matrix (PM) acts as a physical barrier that influences the vector competence of mosquitoes. We have previously shown that gut microbiota promotes PM formation in Anopheles stephensi, although the underlying mechanisms remain unclear. In this study, we identify that the cell wall components of gut commensal bacteria contribute to PM formation. Oral administration of primary cell wall components from both gram-positive and gram-negative bacteria, such as diaminopimelic acid-peptidoglycan (DAP-PGN), lysine-peptidoglycan (Lys-PGN), and lipopolysaccharides (LPS), to mosquitoes, after depleting their gut microbiota with antibiotics, restores the down-regulated expression of the peritrophin1 (Per1) gene, which encodes a structural protein of the PM. Moreover, this administration rescues PM formation upon blood ingestion. PGN and LPS are well-known ligands of innate immune signaling pathways in animals. In mosquitoes, the Toll and IMD (immune deficiency) pathways are the 2 major innate immune signaling pathways. We next knocked down the expression of 2 receptors, Pgrp-s1 and Pgrp-lc, as well as 2 transcription factors, Rel1 and Rel2, which are involved in the Toll and IMD pathways, respectively. Double knockdown of Pgrp-s1 and Pgrp-lc, or Rel1 and Rel2, compromised Per1 expression. Additionally, through dual-luciferase assays and supershift electrophoretic mobility shift assays (EMSAs), we identified a 15-bp binding motif (ATAGACACGAGCACC) for Rel1 and Rel2 in the Per1 promoter region. To further explore the role of individual Toll and IMD pathways in the regulation of Per1 expression, we specifically inhibited the activity of each pathway. While inhibition of the Toll pathway by knocking down Pgrp-s1 or Rel1 did not affect Per1 expression, knockdown of Pgrp-lc or Rel2 in the IMD pathway significantly down-regulated Per1 expression. These findings suggest that the IMD pathway plays a major role in regulating Per1 expression in An. stephensi. In summary, our study uncovers a novel role for bacterial cell wall components in regulating PM formation through activation of mosquito immune signaling pathways.
Citation: Song X, Zhou H, Wang J (2025) Cell wall components of gut commensal bacteria stimulate peritrophic matrix formation in malaria vector mosquitoes through activation of the IMD pathway. PLoS Biol 23(1):
e3002967.
https://doi.org/10.1371/journal.pbio.3002967
Academic Editor: Louis Lambrechts, Institut Pasteur, FRANCE
Received: February 1, 2024; Accepted: December 5, 2024; Published: January 6, 2025
Copyright: © 2025 Song et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This work was supported by the Key Research and Development Program sponsored by The Ministry of Science and Technology (MOST) (2023YFA1801000 to J.W.), the Shanghai Pilot Program for Basic Research – Fudan University (22TQ015 to J.W.) and China Postdoctoral Science Foundation (2023M740700 to X.S.), the Postdoctoral Research Fellowship Program (Grade C) of China Postdoctoral Science Foundation (GZC20230520 to X.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations:
AMP,
antimicrobial peptide; CBD,
chitin-binding domain; CFU,
colony-forming unit; DAP-PGN,
diaminopimelic acid-peptidoglycan; EMSA,
electrophoretic mobility shift assay; LPS,
lipopolysaccharide; Lys-PGN,
lysine-peptidoglycan; PAMP,
pathogen-associated molecular pattern; PBS,
phosphate-buffered saline; PLB,
passive lysis buffer; PM,
peritrophic matrix; PMSF,
phenylmethanesulfonyl fluoride; PRR,
pathogen recognition receptor; RHD,
Rel-homology domain; SDS,
sodium dodecyl sulfate
Introduction
Malaria, which is transmitted through the bites of Anopheles mosquitoes, poses a significant threat to human health. During the infection in mosquitoes, Plasmodium parasites first undergo sexual reproduction in the mosquito midgut, where they develop into ookinetes. These ookinetes then traverse the peritrophic matrix (PM) and epithelial cells and transform into oocysts upon reaching the basal lamina [1]. Throughout this process, PM serves as the first physical barrier encountered by the invading parasites [2–4]. The PM is an acellular layer that forms when mosquitoes ingest a blood meal. It lines the ingested blood bolus and protect the midgut epithelium by separating the blood bolus from the gut epithelial cells [5–7]. The PM is composed of chitin, proteins, and glycoproteins. Chitin serves as the structural scaffold for the PM, providing a platform for proteins to bind [8,9]. Peritrophins, which are mucin linker proteins, play a crucial role in PM formation. They contain a chitin-binding domain (CBD) that enables them to interact with chitin [10,11]. Mucins are glycosylated proteins that lack a CBD domain but still contribute to PM formation by interacting with peritrophins [12].
The PM play distinct roles in influencing pathogen infection in disease-transmitting vectors. In Anopheles mosquitoes, the PM helps defend against Plasmodium infection by blocking the parasite from crossing the midgut epithelium [2]. Elimination or impairment of PM structure increases the susceptibility of Anopheles to Plasmodium infection [13,14]. In Ixodes scapularis, infection with Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis, disrupts the homeostasis of the gut microbiota, leading to impairment of PM structure and facilitating infection [15]. In tsetse flies, newly enclosed adults are more susceptible to trypanosome parasites than mature adults (1 week old) due to the absence of PM [16]. In the sand fly Phlebotomus papatasi, knockdown of PpPer1, a protein involved in PM formation, results in increased Leishmania load [17]. Conversely, it has also been reported that PM protects Leishmania by creating a barrier that prevents proteolytic damage to the parasite in Phlebotomus papatasi [18]. Similarly, the PM in Ixodes scapularis facilitates the colonization of Borrelia burgdorferi, the agent of Lyme disease [19]. Impairment of PM in Aedes aegypti reduces the infectivity of Plasmodium gallinaceum, as well as Zika and dengue virus [20,21]. Although the role of the PM in pathogen infection varies by pathogen, it is pivotal in determining the capacity of vectors to transmit diseases.
The factors influencing PM formation and integrity in most hematophagous vectors remain unclear. Symbiotic bacteria are known to play crucial roles in both stimulating PM formation and maintaining its integrity. In Anopheles mosquitoes and tsetse flies, elimination of symbiotic bacteria through antibiotic treatment compromises PM formation [13,22,23]. In Anopheles mosquito, the gut commensal bacterium Pseudomonas alcaligenes helps maintain PM integrity by degrading the PM-toxic tryptophan metabolite 3-hydroxykynurenine [14]. In ticks, the dysbiosis of the gut microbiota down-regulates STAT expression, leading to reduced expression of PM-related genes [19]. Similarly, in Drosophila, oral administration of gram-negative bacterium Erwinia carotovora 15 induces the expression of drosocrystallin, a chitin-binding protein that contributes to PM formation [24]. Despite these findings, the mechanisms by which bacteria regulate PM formation remain poorly understood.
In this study, we screened the cell components of midgut commensal bacteria in An. stephensi and found that the bacterial cell wall components, PGN and LPS, stimulate the expression of Per1, leading to PM formation. Mechanistic analysis showed that the presence of PGNs/LPS in the midgut activates the IMD and Toll immune signaling pathways. This activation causes NF-κB transcription factors, Rel1 and Rel2, to bind to the Per1 promoter region (−289 bp to −275 bp). Mutation of these 15 nucleotides abolishes the binding capacity of Rel1 and Rel2, thereby blocking Per1 transcription. Furthermore, we demonstrate that the IMD pathway plays a predominant role in stimulating Per1 expression. In summary, our results reveal an uncharacterized role of bacterial cell wall components in stimulating PM formation through immune signaling pathways.
Results
Bacterial cells stimulate PM formation
In our previous studies, we demonstrated that Enterobacter hormaechei, isolated from our colony mosquitoes, has the ability to stimulate PM formation in An. stephensi [22]. To investigate which component of commensal bacterium stimulates PM formation, we administrated mosquitoes, in which gut commensal bacteria were reduced by antibiotics (Abx) treatment, with the supernatant and cell pellets of the overnight culture of E. hormaechei (Fig 1A). The efficacy of bacterial clearance by antibiotics was evaluated by qPCR and plate culture methods (S1 Fig). In mosquitoes fed with E. hormaechei pellets, the bacterial load reached a similar level as that in normal mosquitoes, around 107–108 colony-forming units (CFU), at 45 h after blood feeding. However, the bacterial load in mosquitoes treated with the bacterial supernatant and Abx-treated mosquitoes remained at the same level (Fig 1B). We next analyzed the mRNA and protein levels of Per1, a gene involved in PM formation, in these mosquitoes. Administration of E. hormaechei pellets significantly up-regulated the expression of Per1 mRNA at 24 and 45 h after blood feeding, and Per1 protein at 45 h after blood feeding, reaching levels comparable to those found in the normal mosquito midgut. However, treatment with bacterial supernatant failed to stimulate Per1 expression (Fig 1C and 1D). Consistent with these findings, fully formed PMs were observed by immunofluorescent staining against Per1 and calcofluor staining in mosquitoes treated with bacterial pellets and in normal mosquitoes at 45 h after blood meal, while PM formation was impaired in mosquitoes treated with bacterial supernatant or Abx-treated mosquitoes (Fig 1E and 1F). Based on these results, we conclude that bacterial secretion does not play a role in promoting PM formation. Instead, components present in the bacterial cells are responsible for stimulating PM formation in mosquitoes.
Fig 1. Identification of the effective component of E. hormaechei in promoting PM formation.
(A) Schematic overview of the study design. (B) Bacterial loads in the midgut of normal mosquitoes and mosquitoes with different treatments at 45 h post a blood meal. Abx, antibiotic-treated mosquitoes; Pellets, Abx mosquitoes supplemented with cell pellets of E. hormaechei; Supernatant, Abx mosquitoes supplemented with cell supernatant of E. hormaechei. (C, D) The levels of Per1 mRNA (C) and protein (D) in the midgut of the same treated mosquitoes as in (B). The expression level of Per1 was normalized to S7. The relative expression level of Per1 in treated mosquitoes was normalized to gene expression in Normal mosquitoes. Quantification of Per1 intensity was shown on the panel below in (D). Data are presented as mean ± SEM (n = 5 in B, n = 8~10 in C, and n = 5 in D). (E) Immunofluorescent staining of Per1 (green) in the midgut of the same treated mosquitoes as in (B) at ×200 magnification. Nuclei were stained with DAPI (blue). (F) Calcofluor white staining PM structure in the midgut of the same treated mosquitoes as in (B) at ×200 magnification. Scale bars, 50 μm. Significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. *, P P P S1 Data. The uncropped blots are included in S1 Raw Images. PM, peritrophic matrix.
The bacterial cell wall promotes PM formation
A bacterial cell typically consists of a cell wall that surrounds an internal matrix called the cytoplasm [25]. To identify the bacterial effectors responsible for promoting PM formation, we isolated the bacterial cell wall and intracellular contents from an overnight culture of E. hormaechei. These isolated components were individually administered to Abx-treated mosquitoes through a blood meal (Fig 2A). Supplementation of the bacterial cell wall increased the protein level of Per1, indicating its role in stimulating PM formation. However, the intracellular contents had no influence on Per1 protein level (Fig 2B).
Fig 2. Identification of the bacterial cell component in PM formation.
(A) Schematic overview of the study design. (B) Western blot of Per1 in the midguts of normal, Abx mosquitoes and Abx mosquitoes treated with cell wall and cell content at 45 h post a blood meal. (C–E) The expression levels of Per1 of mosquitoes orally supplemented with serial concentrations of DAP-PGN (C), Lys-PGN (D), LPS (E), at 24 and 45 h post a blood meal. (F, G) The expression levels of Per1 mRNA (F) and protein (G) in the midgut of Normal, Abx, and Abx mosquitoes treated with the mixture of DAP-PGN, Lys-PGN, and LPS (PGNs+LPS) at 24 and 45 h post a blood meal. The expression level of the target gene was normalized to S7. The relative expression level of Per1 was normalized to gene expression in Abx or Normal mosquitoes. Quantification of Per1 intensity was shown on the right panel in (G). Data are presented as mean ± SEM (n = 8~10 in C, n = 7~10 in D, n = 8~10 in E, and n = 10 in F and n = 4 in G). (H) Immunofluorescent staining of Per1 (green) in midguts of Normal, Abx, and PGNs+LPS treated mosquitoes at 45 h post a blood meal at ×200 magnification. Nuclei were stained with DAPI (blue). (I) Calcofluor white staining PM structure in the midgut of Normal, Abx, and PGNs+LPS treated mosquitoes at 45 h post a blood meal at ×200 magnification. Scale bars, 50 μm. Significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. *, P P P P S1 Data. The uncropped blots are included in S1 Raw Images. DAP-PGN, diaminopimelic acid-peptidoglycan; LPS, lipopolysaccharide; Lys-PGN, lysine-peptidoglycan; PM, peritrophic matrix.
Peptidoglycan, including DAP-PGN and Lys-PGN, as well as LPS, are major components of the bacterial cell wall [26]. We next fed Abx-treated mosquitoes with blood containing varying concentrations of DAP-PGN, Lys-PGN, or LPS individually, and analyzed Per1 expression levels of at 24 h and 45 h post-blood feeding. All 3 components stimulated Per1 expression at 24 h post-blood feeding, while DAP-PGN continued to elevate Per1 expression at 45 h (Fig 2C–2E), suggesting that DAP-PGN elicits a more sustained induction of Per1 than other cell wall components. Since the induction of Per1 by LPS may be due to the PGN contamination [27,28], we next treated LPS with mutanolysin, a PGN lytic enzyme [27], and then fed mosquitoes with the treated LPS via blood. Again, significant up-regulation of Per1 expression was observed in both mutanolysin-untreated and treated LPS mosquitoes (S2 Fig), suggesting that LPS is involved in the stimulation of PM formation. Considering that the mosquito gut microbiota consists of both gram-negative and gram-positive bacteria [29], we combined all 3 cell wall components and fed the mixture to Abx-treated mosquitoes via blood. As expected, oral administration of the PGNs/LPS mixture restored the mRNA and protein levels of Per1 to similar levels as those observed in normal mosquitoes (Fig 2F and 2G). Additionally, intact PM formation was observed in the midgut of mosquitoes orally supplemented with the mixture (Fig 2H and 2I). Taken together, these results indicate that the components of the bacterial cell wall, including DAP-PGN, Lys-PGN, and LPS, play a crucial role in stimulating PM formation.
The PGNs/LPS mixture stimulates Per1 expression prior to blood feeding
Peritrophins are stored in the secretory vesicles of midgut epithelial cells before blood feeding and are released into the gut lumen immediately after mosquitoes ingest a blood meal [30–32]. To investigate whether the elimination of gut commensal bacteria influences Per1 level before a blood meal, we analyzed the expression level of Per1 in mosquitoes that were only fed sugar (Fig 3A). As expected, the mRNA and protein levels of Per1 were reduced significantly in Abx-treated mosquitoes compared to normal mosquitoes (Fig 3B and 3C). However, when we administered the PGNs/LPS mixture to Abx-treated mosquitoes via a sugar meal, we found that the mixture induced Per1 expression at both the mRNA and protein levels even without a blood meal (Fig 3B and 3C). Additionally, 2 other PM-related genes were also regulated by bacterial cell wall components (S3 Fig) [33–35]. These results suggest that the PGNs/LPS mixture stimulates PM genes expression independently of the type of food that the mosquito ingests. Even in the absence of a blood meal, the administration of the PGNs/LPS mixture can trigger PM gene expression, indicating that the bacterial cell wall components play a direct role in stimulating PM formation in the mosquito gut.
Fig 3. The influence of PGNs and LPS on Per1 expression before blood feeding.
(A) Schematic overview of the study design. (B, C) The levels of Per1 mRNA (B) and protein (C) in the midgut of Normal, Abx, Abx mosquitoes treated with DAP-PGN/Lys-PGNs/LPS mixture via sugar meal for 24 h. The expression level of Per1 was normalized to S7. The relative expression levels of Per1 in Abx, Abx supplemented with PGNs+LPS mosquitoes were normalized to gene expression in Normal mosquitoes. Quantification of Per1 intensity was shown on the right panel in (C). Data are presented as mean ± SEM (n = 7~10 in B, n = 4 in C). Significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. *, P P P S1 Data. The uncropped blots are included in S1 Raw Images. DAP-PGN, diaminopimelic acid-peptidoglycan; LPS, lipopolysaccharide; Lys-PGN, lysine-peptidoglycan.
Per1 expression is regulated by IMD and Toll pathways in mosquito
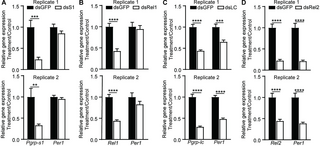
The cell wall components, particularly PGNs and LPS, are well-known activators of the mosquito innate immune system [36–38]. We hypothesized that the bacterial cell wall might regulate Per1 expression through immune signaling pathways. In Drosophila, the peptidoglycan recognition proteins PGRP-LC and -SA are responsible for the recognition of PGNs and act as receptors for the IMD and Toll pathways, respectively [39–42]. In mosquitoes, PGRP-LC is the key receptor of IMD pathway [43], while PGRP-S1 is a putative receptor of Toll pathway [44–46]. To investigate whether the IMD and Toll pathways regulate Per1 expression through sensing PGN and LPS, we conducted a co-silencing experiment targeting Pgrp-lc and –s1 (dsLC/S1) and examined the expression level of Per1 in mosquitoes before blood feeding. The mRNA and protein levels of Per1 were significantly reduced in mosquitoes with Pgrp-lc and Pgrp-s1 double knockdown compared to those with dsGFP controls (Fig 4A and 4B). As expected, the knockdown of Pgrp-lc and Pgrp-s1 inhibited the activities of the IMD and Toll signaling pathways, as indicated by the significantly reduced expression levels of antimicrobial peptides (AMPs), including Cecropin3, Gambicin, and Defensin (Fig 4A). The simultaneous decrease in the expression of Per1 and AMPs in dsLC/S1 mosquitoes suggests that these genes may be regulated by common transcription factors or signaling components downstream of the IMD and Toll pathways.
Fig 4. The influence of Toll and IMD pathways on Per1 expression.
(A) Relative expression level of Per1 and immune related genes in mosquitoes treated with dsLC/S1 and dsGFP (n = 10). (B) Western blot of Per1 in the midgut of dsGFP and dsLC/S1-treated mosquitoes. Quantification of Per1 intensity was shown on the right panel in (B). (C) The knocking down efficiency of Rel1 and Rel2 of mosquitoes (dsRel1/Rel2). (D, E) The levels of Per1 transcript (D) and protein (E) in dsRel1/Rel2 and dsGFP-treated mosquitoes prior to blood feeding. Quantification of Per1 intensity was shown on the right panel in (E). (F, G) The levels of Per1 transcript (F) and protein (G) in dsRel1/Rel2 and dsGFP-treated mosquitoes at 45 h post blood feeding. The expression levels of the target genes were normalized to S7. The relative expression level of immune genes in dsRel1/Rel2-treated mosquitoes was normalized to gene expression in dsGFP-treated mosquitoes. Quantification of Per1 intensity was shown on the right panel in (G). (H) The total gut microbiota load was measured in dsRel1/Rel2 and dsGFP treated mosquitoes prior to blood feeding. All Data are presented as mean ± SEM (n = 9~10 in A, n = 3 in B, n = 8~9 in C, n = 8~9 in D, n = 4, n = 9~10 in F, n = 5 in G, n = 8~10 in H). Significance was determined by Student’s t test. The microbiota load was determined by Mann–Whitney test. *, P P P P S1 Data. The uncropped blots are included in S1 Raw Images.
The NF-κB transcription factors Rel1 and Rel2, which belong to the Toll and IMD pathways, respectively, are the primary regulators of downstream immune effector expression. To investigate whether Rel1 and Rel2 regulate Per1 expression, we conducted a double knockdown experiment targeting Rel1 and Rel2 and analyzed Per1 expression. When Rel1 and Rel2 were simultaneously knocked down, both the mRNA and protein levels of Per1 were significantly reduced compared to the dsGFP control, regardless of whether mosquitoes were fed sugar or blood (Fig 4C–4G). Moreover, the expression levels of Per14 and Fibrinogen, which were induced by treatment with PGNs/LPS mixture in Abx mosquitoes, were also reduced in dsRel1/Rel2 mosquitoes (S4 Fig). Given that the NF-κB signaling pathways are responsible for controlling the abundance of gut microbiota that stimulates PM formation, we investigated whether the down-regulation of Per1 expression was due to alterations in gut bacteria abundance. The bacterial load in the midgut of dsRel1/Rel2 mosquitoes was significantly increased prior to and post blood meal due to the inhibition of mosquito Toll and IMD signaling activities (Figs 4H and S4C). However, despite the increased bacterial load, the expression level of Per1 in these mosquitoes remained down-regulated, further confirming that the inhibiting Rel1 and Rel2 activity prevents the initiation of Per1 expression. These results suggest that Rel1 and Rel2 directly regulate Per1 expression and underscore the importance of these NF-κB transcription factors in coordinating the immune response and PM formation in mosquitoes.
Rel1/Rel2 control the expression of Per1 by binding to a 15 bp regulatory motif
Mosquito Rel1 and Rel2 contain the Rel-homology domain (RHD), which initiates the transcription of target genes. We next co-expressed the RHD domains of Rel1 and Rel2, respectively, and investigated their activity on Per1 transcription using a dual-luciferase reporter assay. The pCMV6-Rel1-RHD and pCMV6-Rel2-RHD plasmids were co-transfected into 293T cells. The pGL3-Basic Reporter plasmids containing promoter fragment 1,589 bp upstream of the Per1 coding sequence was transfected into 293T cells (Fig 5A). The plasmid that constitutively expresses renilla luciferase (pRLTK-renilla) was co-transfected as an internal control. At 48 h post-transfection, Rel1 and Rel2 drove the expression of the firefly luciferase reporter successfully in the presence of the −1,589 bp promoter region (Fig 5B). We next analyzed the Rel1 and Rel2 recognition region by transfecting cells with different lengths of promoter fragments ranging from −1,589 to −229 bp upstream of the coding sequence. We found that the deletion of −289 to −229 bp resulted in the loss of capability to activate the firefly luciferase reporter expression, compared to transfection with the pGL3-Basic Reporter vector (Fig 5B). This result indicates that the binding motif of Rel1 and Rel2 may exist within the −289 bp to −229 bp region.
Fig 5. Identification of the Rel1/Rel2 binding motif in Per1 promoter.
(A) A diagram of Per1 promoter and the truncated constructs used in the transient transfection assays. The truncations of the Per1 promoter, which were sequentially truncated from the 5′-end of promoter region, derived from the −1,589 bp to −229 bp, were inserted into pGL3-Basic vector. The inserted promoters were followed by a firefly luciferase gene (green), thereby enabling the determination of the regulatory activity of the inserted promoters using dual luciferase reporter assay. The pGL3-Basic vector was function as control named as pGL3-NC. (B) Assessment of promoter activity for Per1 promoter truncations together with Rel1-RHD and Rel2-RHD plasmids. (C) Schematic representation of M1, M2, M3, M4 mutants, derived from the −289 bp to −229 bp in the Per1 promoter. M, mutation. (D) Assessment of promoter activity for Per1 promoter mutations together with Rel1-RHD and Rel2-RHD plasmids. The activity of Per1 promoter with different mutations was normalized to the activity of the control luciferase construct. Each experiment was performed at least 2 times independently and in duplicate. Data are presented as mean ± SEM. Significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. *, P P P Per1 promoter by EMSA. The arrows indicate specific DNA-protein complex. The data underlying this figure can be found in S1 Data. The uncropped blots are included in S1 Raw Images. EMSA, electrophoretic mobility shift assay; RHD, Rel-homology domain.
To identify binding motifs within the minimal 61 bp region, we constructed 4 deletion mutants from −289 bp to −229 bp of the Per1 promoter sequence and designated them as M1 (−289 bp to −275 bp deletion), M2 (−274 bp to −260 bp deletion), M3 (−259 bp to −245 bp deletion), and M4 (−244 bp to −229 bp deletion), respectively (Fig 5C). Only M1 failed to activate firefly luciferase reporter expression, compared to wild-type controls (pGL3-489), indicating that the binding motif is located at the region from −289 bp to −275 bp of Per1 promoter (Fig 5C and 5D). To further validate this, we performed an electrophoretic mobility shift assay (EMSA). The pCMV6-Rel1-RHD and pCMV6-Rel2-RHD plasmids containing His-tag were co-transfected into 293T cells. When biotin-labeled probes were incubated with cell lysates, a shifted band was observed, indicating that Rel1 and Rel2 proteins bind to biotin-labeled probes. The band intensities gradually decreased with the addition of 5-fold, 50-fold, 100-fold, and 200-fold excess of unlabeled competitive oligonucleotide probes, suggesting that Rel1 and Rel2 proteins binding to probes specifically. When a mutant biotin-labeled probe deleted of −289 bp to −275 bp region was added, the shifted band was disappeared (Fig 5E and 5F). However, we observed a band larger than the shift band possibly due to the nonspecific binding of the mutant probe to the cell lysate. Furthermore, a super-shift band that represents the DNA-protein complex was detected when anti-His antibody was added, indicating that there is one binding site on the Per1 promoter for Rel1 and Rel2.
The IMD pathway plays a major role in regulating Per1 expression
As Toll and IMD pathways play different roles in maintaining gut microbial homeostasis and defending against pathogens [47], we next investigated the individual contributions to PM formation. We knocked down Pgrp-s1, Pgrp-lc, Rel1, and Rel2 individually and analyzed Per1 expression levels (Fig 6). Knockdown of Pgrp-s1 or Rel1, associated with the Toll pathway, had no effect on Per1 expression (Fig 6A and 6B). In contrast, specific knockdown of Pgrp-lc and Rel2, key components of the IMD pathway, significantly down-regulated Per1 expression (Fig 6C and 6D). Consistent with the stronger effect of DAP-PGN on Per1 expression (Fig 2C), these findings strongly suggest that IMD pathway plays a major role in regulating PM formation.
Fig 6. The impact of single gene knockdown in Per1 expression.
The gene knockdown efficiency and Per1 expression levels in Pgrp-s1 (A), Rel1 (B), Pgrp-lc (C), and Rel2 (D) knockdown mosquitoes. Data are presented as mean ± SEM (n = 9~10 in A, n = 10 in B, n = 10 in C, n = 8~10 in D). Significance was determined by Student’s t test. **, P P P S1 Data.
Discussion
The PM, similar to mammalian gut mucus, acts as a physical barrier that protects the insect midgut epithelium from damage. Symbiotic bacteria in hematophagous vectors, such as mosquitoes, ticks, and tsetse flies, play important roles in maintaining PM integrity; however, the underlying mechanisms remain unclear [19,22,23]. Our study revealed that PGNs and LPS are key regulators of PM formation. In the mosquito midgut, the recognition of LPS and PGNs by PGRPs triggers the activation of innate immune signaling pathways. Among these pathways, the IMD pathway plays a predominant role in stimulating PM formation by initiating Per1 expression, which is essential for PM development.
The bacterial cell wall contains typical pathogen-associated molecular patterns (PAMPs) that activate host immune responses through pathogen recognition receptors (PRRs) [44,48]. For example, DAP-PGN, mostly derived from gram-negative bacteria triggers the IMD pathway that results in Relish activation and robust gene expression of antimicrobial peptide in Drosophila [27,49]. While Lys-PGN, derived from gram-positive bacteria, has a higher affinity to PGRP-SA and activates the Toll pathway [50]. In addition, LPS on the surface of gram-negative bacteria cell wall also activates the Drosophila immune system, albeit with weaker effects [50]. Beyond immune activation, PGN and LPS also play critical roles in regulating insect physiology. For example, DAP-PGN modulates Drosophila female egg-laying behavior by activating the NF-κB pathway in octopaminergic neurons [51–53]. Systemic infection with PGN derived-from Pseudomonas aeruginosa reduces the sperm viability of Drosophila melanogaster [54]. LPS suppresses feeding and egg-laying behaviors in Drosophila to prevent infection by stimulating gustatory neurons via a TRPA1-dependent cation channel [55,56]. Furthermore, LPS induces grooming behavior by activating wing chemoreceptors, helping flies defend against pathogens and parasites [57]. In this study, we demonstrate a novel role of PGN and LPS in stimulating PM formation, thereby enhancing physical barrier function in mosquitoes. These findings suggest that, in addition to acting as PAMPs, LPS and PGN serve as signaling molecules regulating various physiological processes in insects. However, this study focused exclusively on typical cell wall components, PGNs and LPS, in PM formation. Investigating the roles of other bacterial cell wall components, such as teichoic acid, lipoteichoic acid, or unusual structural components, in PM formation will be an important area for future research.
The Toll and IMD pathways are key immune signaling pathways that protect insects from pathogens infection and regulate gut microbiota homeostasis through NF-κB transcription factors [47,58]. In mosquitoes, Rel1, a homolog of Drosophila Dorsal, function as a Toll pathway transcription factor, regulating the synthesis of immune effectors [59]. Rel2, the mosquito equivalent of Drosophila Relish governs the IMD pathway [60–62]. Unlike Drosophila IMD pathway that primarily responds to gram-negative bacteria [63], the mosquito IMD pathway responds to both gram-positive and gram-negative bacteria [47] and serves as the predominant immune pathway in the midgut [64]. This pathway also plays a crucial role in Plasmodium defense in mosquitoes. Suppressing IMD activity significantly increases mosquito susceptibility to Plasmodium infection, while its activation reduces susceptibility in Anopheles mosquitoes [43,61,65].
Beyond their canonical roles in immune regulation, the IMD and Toll pathways also regulate other essential physiological processes in Drosophila. For instance, the Toll pathway contributes to epithelial wound repair by controlling E-cadherin expression, a critical component of adherens junctions [66]. It also plays a key role in regulating aging and lifespan in Drosophila neural cells [67]. Similarly, the IMD pathway is involved in salivary gland degradation during Drosophila larval development by regulating autophagy [68]. In this study, we demonstrate that the IMD pathway plays a predominant role in contributing to PM formation by regulating Per1 expression, which is consistent with its major role in immune response in the midgut. Additionally, Per1 has been reported to be regulated by the JAK/STAT signaling pathway in Ixodes ticks [19]. Given the diversity of gut microbiota, it is plausible that multiple signaling pathways collectively regulate the expression of peritrophin genes, thereby maintaining PM integrity.
In this study, we focused exclusively on the influence of bacteria on PM formation. Given that the PM plays diverse roles in mosquito physiology, such as facilitating blood digestion, defending against pathogens, and maintaining homeostasis [5,69], further research is needed to explore how bacteria-mediated PM formation impacts mosquito biology. In summary, our findings uncover the molecular basis of a noncanonical regulatory role for bacterial cell wall components, LPS and PGNs, in promoting PM formation. Additionally, we highlight an atypical role for immune signaling pathways in maintaining gut barrier integrity. These insights provide a foundation for understanding the intricate interactions between gut microbiota and mosquito physiology.
Materials and methods
Mosquito rearing and antibiotic treatment
An. stephensi (Strain Hor) larvae were reared in water that was supplemented with fish food daily. Adult mosquitoes were kept in a controlled environment at 28°C, 80% relative humidity and at a 12 h light/dark cycle according to standard procedures [70,71]. They were provided with a 10% sugar solution and BALB/c mice for blood feeding. For antibiotic treatment, newly emerged mosquitoes were given fresh filtered 10% sucrose containing 10 U/ml penicillin, 10 μg/ml streptomycin, and 15 μg/ml gentamicin daily for up to 5 days. To determine the efficacy of antibiotic treatment, mosquitoes were surface sterilized with 75% ethanol twice and 0.9% NaCl twice, then homogenized in 0.9% NaCl. The homogenate was plated on the LB agar plates. CFU were counted after incubating the plates at 28°C for 2 days and 5 days. The genomic DNA was extracted using the method of Holmes and Bonner as described [72] and the bacteria load was determined by qPCR using universal 16S rRNA primers (S1 Table). After removing antibiotics, the mosquitoes were reared in sterilized cages and provided with sterilized sucrose solution to maintain a sterile environment [73].
Bacterial cell wall purification
The purification procedure of bacterial cell wall was performed as described [74]. Briefly, the E. hormaechei was grown in LB medium at 28°C overnight. Then, 1 × 107–108/ml bacterial cells were harvested by centrifugation at 5, 000 g for 10 min at 4°C. The pellets were collected, washed with distilled endotoxin-free water, and boiled in 8% sodium dodecyl sulfate (SDS) (Sigma-Aldrich) for 30 min. Then, the cells were incubated at room temperature overnight. Afterward, they were centrifuged at 25,000 g for 20 min at 4°C. The resulting cell pellets were resuspended in 4% SDS and boiled for 15 min. Following this, the cell wall was harvested by centrifugation at 25,000 g for 20 min at 4°C and resuspended in distilled endotoxin-free water. This process was repeated 3 times. Finally, the pellets were resuspended in 2 M NaCl and collected by centrifugation at 25,000 g for 20 min at 4°C. The supernatant was discarded and the remaining pellets were dissolved in 10% sucrose or blood for oral feeding mosquitoes.
Bacterial contents preparation
Briefly, the E. hormaechei was grown in LB medium at 28°C overnight. Then, 1 × 107–1 × 108/ml bacterial cells were harvested by centrifugation (5,000 g, 10 min, 4°C). The supernatant was discarded, and the cell pellets were washed 3 times with sterile 1× phosphate-buffered saline (1× PBS). The cell pellets were then resuspended in PBS and sonicated on ice in the presence of protease inhibitor (Beyotime). Cell contents were obtained by centrifugation at 143,000 g for 1 h at 4°C and filtered through a 0.22 μm membrane. Then, the filtrate was used for oral administration to mosquitoes via blood meal [75,76].
Oral administration cell components of bacteria
The oral administration of E. hormaechei was performed as described [77]. Briefly, the overnight culture E. hormaechei was harvested by centrifugation (5,000 g, 10 min, 4°C). The pellet and the supernatant were collected. The pellet was washed 2 times with PBS and dissolved in 1.5% sucrose to get the final concentration 1 × 107–108/ml. The supernatant was filtered through a 0.22 μm membrane before adding to 1.5% sucrose. The commercial Lys-PGN derived from Staphylococcus aureus (Sigma-Aldrich), DAP-PGN derived from Bacillus subtilis (Sigma-Aldrich), and LPS derived from E. coli (Sigma-Aldrich) were dissolved in sterile water and were administrated to antibiotics treated mosquitoes through a blood meal or 10% sucrose at dedicated concentration. To eliminate the PGN contamination in LPS, the LPS (1 mg/ml) (Sigma) were incubated with mutanolysin (Sigma) at 22°C for 12 h with shaking [78]. The mutanolysin-treated LPS was then orally administrated to Abx-treated mosquitoes through an artificial membrane-feeding system (Hemotek). The mosquito midgut was dissected at 24 h post blood feeding, and the gene expression of Per1 was analyzed by qPCR.
Immunofluorescence analysis
The immunofluorescence analysis of Per1 was performed as previously described [14]. Briefly, mosquito abdomens were collected 45 h after a blood meal and fixed in 4% paraformaldehyde at 4°C overnight. Samples were paraffin-embedded, sectioned at thickness of 5 μm, and stained with anti-Per1 (1:100) and Alexa Fluor 546 (1:5,000) (Thermo Fisher). Images were captured using a Nikon ECLIPSE IVi microscope connected to a Nikon DIGITAL SIGHT DS-U3 digital camera.
Calcofluor white staining
Briefly, mosquito abdomens were collected 45 h after a blood meal and fixed in 4% paraformaldehyde at 4°C overnight. Samples were embedded in paraffin and sectioned at 5 μm thickness. After deparaffinization, the sections were washed 3 times with PBS and then stained with calcofluor white (Sigma) at room temperature for 30 min in the dark. After staining, the sections were rinsed 3 times with distilled water. The sections were then dried and images were captured using a Nikon ECLIPSE IVi microscope connected to a Nikon DIGITAL SIGHT DS-U3 digital camera.
RNA isolation, cDNA synthesis, and quantitative PCR (RT-qPCR)
Total RNA was extracted from individual mosquitoes using the standard TRIzol reagent (Sigma-Aldrich). The cDNA was synthesized using the Hifair III 1st Strand cDNA Synthesis SuperMix for qPCR (Yeasen). The expression level of the target genes were determined by qPCR using a Roche LightCycler 96 Real Time PCR Detection System with SYBR Green qPCR Master Mix (Yeasen). Data were processed and analyzed using LightCycler 96 software. The ribosomal gene S7 of An. stephensi was used as an internal reference [79]. The relative expression of the target gene was normalized to S7 by the 2-△△Ct method [80]. Seven to 10 mosquitoes were used for qPCR analysis each time. Primers were listed in supplementary S1 Table.
Western blot analysis
Mosquito midguts were dissected at 45 h post-blood meal and at least 10 midguts were pooled together. Proteins were extracted using lysis buffer (8 M urea, 2% SDS, 5% β-mercaptoethanol, 125 mM Tris-HCl). Immunoblotting was performed using standard procedures with rabbit anti-Per1 polyclonal antibody (1:5,000) [81], anti-Actin monoclonal antibody (1:2,000) (Abbkine), and secondary anti-rabbit-HRP (1:5,000) (Abbkine). Actin was used as a reference control [14,81]. Intensity of the signals was quantified by ImageJ software [82].
RNA interference
The cDNA clones of target genes were obtained using gene-specific primers (S1 Table). PCR amplicons of GFP (as a control) (BD Biosciences), Pgrp-lc (ASTE016447), Pgrp-s1 (ASTE007708), Rel1 (ASTE011378), Rel2 (ASTE010360) tailed with a T7 promoter (TAA TAC GAC TCA CTA TAG GGA GA) were used to synthesize dsRNA using a MEGAscript T7 High Yield Transcription Kit (Invitrogen). Four- to 5-day-old females were received a total of 69 nL dsRNA (3 to 4 μg/μl), which was injected intra-thoracically using a nanoject II microinjector (Drummond). Gene silencing efficiency was determined 2 days after injection. All experiment was replicated twice with separate cohorts of mosquitoes.
Plasmid construction
The promoter sequence, located 1,589 bp upstream of the Per1 coding sequence, and the different truncations of the An. stephensi Per1 promoter were amplified using the primers listed in S1 Table. The resulting PCR products were then cloned into plasmid pGL3-Basic vectors carrying the firefly luciferase reporter (Promega). The RHD of Rel1 and Rel2 were amplified from an An. stephensi cDNA using the primers listed in S1 Table, and then cloned into pCMV6-AC-HA vectors (OriGene), named as pCMV6-Rel1-RHD, and pCMV6-Rel2-RHD, respectively. The pRL-TK plasmid carrying renilla luciferase were used as an internal control (Promega). The 4 mutations of the Per1 promoter (M1-M4) were constructed by amplifying the 489 bp promoter region (pGL3-489) using the primers listed in S1 Table. Plasmids for transfection were prepared using the EndoFree Maxi Kit Plasmid Kit (TIANGEN).
Dual luciferase assay
Approximately 0.5 million 293T cells were seeded per well in 24-well plates. Once the cells reached almost 70% confluence, the pGL3-Per1, PCMV6-Rel1-RHD-His, pCMV6-Rel2-RHD-His plasmids, and pRL-TK plasmids were transfected into the 293T cells using Hieff Trans Liposomal Transfection Reagent (Yeasen). To investigate the influence of Rel2 and Rel1 on Per1 expression, the pGL3-Per1, PCMV6-Rel1-RHD-His, pCMV6-Rel2-RHD-His, and pRL-TK plasmids were transfected into the 293T cells. At 48 h post transfection, cells were harvested and lysed with 100 μl per well passive lysis buffer (PLB) supplied with the Dual-Luciferase Report System Kit (Promega). The lysates were centrifuged, and the supernatant was transferred into a 96-well white polystyrene assay plate (Corning). The firefly and renilla luciferase signals were detected according to the manufacturer’s instructions for the Dual Luciferase Report Assay System (Promega) using the GloMax 96 Microplate Luminometer (Promoga). The value of firefly luciferase was normalized by that of renilla luciferase.
Electrophoretic mobility shift assay (EMSA)
The 293T cells were transfected with a 1:1 mix of pCMV6-Rel1-RHD-His and pCMV6-Rel2-RHD-His plasmids. After 48 h, the transfected cells were harvested and lysed using 200 μl of cell lysis buffer (Beyotime) containing protease inhibitor PMSF (phenylmethanesulfonyl fluoride) (Beyotime) with the following components: 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, sodium pyrophosphate, β-glycerophosphate, EDTA, and Na3VO4. The lysates were centrifuged at 1,3000 rpm for 15 min at 4°C. The resulting supernatant was transferred to a clean, pre-chilled tube and stored at −80°C until use. The EMSA assay was performed using a Light Shift chemiluminescent EMSA Kit (Thermo) according to the manufacturer’s instructions. Briefly, the protein extracts, biotin-labeled oligonucleotide probes and anti-His antibody (Abmart), were added to a 20 μl reaction system containing 1× binding buffer (1 mM Tris, 50 mM KCl, 1 mM DTT; pH 7.5), 0.05% NP-40, 2.5% glycerol, and 50 ng/μl poly (dI:dC). Unlabeled or mutant oligonucleotides were used as the competitors. After incubating at room temperature for 20 min, the reaction mixture was run on a 5% polyacrylamide gel, and then transferred to a Nylon membrane (Bio-Rad) using a Transblot R SD Simi-Dry Transfer System (Bio-Rad). The DNA was then cross-linked to the membrane for 15 min. The membrane was incubated in 20 ml of blocking buffer for 15 min. Subsequently, it was replaced it with another 20 ml of blocking buffer containing 66.7 μl of stabilized streptavidin HRP conjugate (1:300 dilution) for 15 min. After washing 4 times, the membrane was transferred to a new container with 30 ml of substrate equilibration buffer and incubated for 5 min. After being treated with a substrate working solution provided by the EMSA kit, the membrane was imaged using a ChemiDocTM Imaging System (Bio-Rad).
Statistical analysis
All statistical analyses were performed using GraphPad Prism software (v.8). Details of statistical methods were described in the figure legends. Differences in gene expression between 2 groups were analyzed using the Student’s t test, or using analysis of one-way ANOVA followed by Dunnett’s multiple comparison test for more than 2 groups. The microbiota levels of mosquitoes between 2 groups were analyzed using the Mann–Whitney test or using analysis of one-way ANOVA followed by Dunnett’s multiple comparison test for more than 2 groups.
Supporting information
S1 Fig. Bacterial clearance efficacy in An. stephensi.
(A) Quantification of midgut bacterial loads by qPCR in the normal (Normal) and antibiotic-treated (Abx) mosquitoes 5 days after antibiotic treatment. (B) CFU of midgut bacteria in normal (Normal) and antibiotic-treated (Abx) mosquitoes growing on LB plates for 5 days. Data are presented as mean ± SEM (n = 10 in A, n = 10 in B). Significance was determined by Mann–Whitney test. ****, P S1 Data.
https://doi.org/10.1371/journal.pbio.3002967.s001
(TIF)
S2 Fig. The influence of LPS on Per1 expression.
The LPS was treated with mutanolysin and orally supplemented to mosquitoes. The mutanolysin-untreated LPS and mutanolysin-supplemented mosquitoes were used as controls. Data are presented as mean ± SEM (n = 10). Significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. **, P S1 Data.
https://doi.org/10.1371/journal.pbio.3002967.s002
(TIF)
S3 Fig. The influence of DAP-PGN/Lys-PGN/LPS mixture on other PM genes expression.
(A, B) The expression level of Per14 (A) and Fibrinogen (B) in the midgut of Normal, Abx, Abx mosquitoes treated with DAP-PGN/Lys-PGN/LPS (PGNs+LPS) for 24 h via sugar meal. Data are presented as mean ± SEM (n = 10 in A, n = 10 in B). Significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. *, P P S1 Data.
https://doi.org/10.1371/journal.pbio.3002967.s003
(TIF)
S4 Fig. PM gene and microbiota regulation by Rel1 and Rel2.
The expression levels of Per14 (A) and Fibrinogen (B) in dsRel1/Rel2 and dsGFP-treated mosquitoes prior to blood feeding. (C) The total gut microbiota load was measured in dsRel1/Rel2 and dsGFP-treated mosquitoes post blood feeding. Data are presented as mean ± SEM (n = 8~9 in A and B, n = 8~10 in C). Significance was determined by Student’s t test in A and B and by Mann–Whitney test in C. *, P P S1 Data.
https://doi.org/10.1371/journal.pbio.3002967.s004
(TIF)
Acknowledgments
We thank Dr. Hongyan Wang from Obstetrics and Gynecology Hospital, State Key Laboratory of Genetic Engineering at School of Life Sciences, Key Laboratory of Reproduction Regulation of NPFPC, Institute of Reproduction and Development, Fudan University for providing pCMV6-AC-HA vector. We thank Dr. Jianfeng Dai from Institute of Biology and Medical Sciences, Jiangsu Key Laboratory of Infection and Immunity, Soochow University for providing pGL3-Basic and pRL-TK vector.
References
- 1.
Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol. 2005;21(12):573–580. WOS:000234320300011. pmid:16236552 - 2.
Abraham EG, Jacobs-Lorena M. Mosquito midgut barriers to malaria parasite development. Insect Biochem Mol Biol. 2004;34(7):667–671. pmid:15242707. - 3.
Sinden RE, Billingsley PF. Plasmodium invasion of mosquito cells: hawk or dove? Trends Parasitol. 2001;17(5):209–212. pmid:11323288. - 4.
Whitten MMA, Shiao SH, Levashina EA. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol. 2006;28(4):121–130. WOS:000236064500002. pmid:16542314 - 5.
Erlandson MA, Toprak U, Hegedus DD. Role of the peritrophic matrix in insect-pathogen interactions. J Insect Physiol. 2019;117:103894. pmid:31175854. - 6.
Aksoy S. Tsetse peritrophic matrix influences for trypanosome transmission. J Insect Physiol. 2019;118:103919. pmid:31425686; PubMed Central PMCID: PMC6853167. - 7.
Hegedus DD, Toprak U, Erlandson M. Peritrophic matrix formation. J Insect Physiol. 2019;117:103898. pmid:31211963. - 8.
Tellam RL, Vuocolo T, Johnson SE, Jarmey J, Pearson RD. Insect chitin synthase cDNA sequence, gene organization and expression. Eur J Biochem. 2000;267(19):6025–6043. pmid:10998064. - 9.
Clarke L, Temple GH, Vincent JF. The effects of a chitin inhibitor-dimilin- on the production of peritrophic membrane in the locust, Locusta migratoria. J Insect Physiol. 1977;23(2):241–246. pmid:323371 - 10.
Shen Z, Jacobs-Lorena M. A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin. Cloning, expression, and characterization. J Biol Chem. 1998;273(28):17665–17670. pmid:9651363. - 11.
Agrawal S, Kelkenberg M, Begum K, Steinfeld L, Williams CE, Kramer KJ, et al. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem Mol Biol. 2014;49:24–34. pmid:24680676. - 12.
Hegedus D, Erlandson M, Gillott C, Toprak U. New Insights into Peritrophic Matrix Synthesis, Architecture, and Function. Annu Rev Entomol. 2009;54:285–302. WOS:000262482300016. pmid:19067633 - 13.
Rodgers FH, Gendrin M, Wyer CAS, Christophides GK. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog. 2017;13(5). ARTN e1006391 WOS:000402877700040. pmid:28545061 - 14.
Feng Y, Peng Y, Song X, Wen H, An Y, Tang H, et al. Anopheline mosquitoes are protected against parasite infection by tryptophan catabolism in gut microbiota. Nat Microbiol. 2022;7(5):707–715. pmid:35437328. - 15.
Abraham NM, Liu L, Jutras BL, Yadav AK, Narasimhan S, Gopalakrishnan V, et al. Pathogen-mediated manipulation of arthropod microbiota to promote infection. Proc Natl Acad Sci U S A. 2017;114(5):E781–E790. WOS:000393196300016. pmid:28096373 - 16.
Haines LR. Examining the tsetse teneral phenomenon and permissiveness to trypanosome infection. Front Cell Infect Microbiol. 2013;3:84. pmid:24312903; PubMed Central PMCID: PMC3833344. - 17.
Coutinho-Abreu IV, Sharma NK, Robles-Murguia M, Ramalho-Ortigao M. Characterization of Phlebotomus papatasi Peritrophins, and the Role of PpPer1 in Leishmania major Survival in its Natural Vector. PLoS Neglect Trop D. 2013;7(3). ARTN e2132 WOS:000316943800045. pmid:23516661 - 18.
Pimenta PF, Modi GB, Pereira ST, Shahabuddin M, Sacks DL. A novel role for the peritrophic matrix in protecting Leishmania from the hydrolytic activities of the sand fly midgut. Parasitology. 1997;115(Pt 4):359–369. pmid:9364562. - 19.
Narasimhan S, Rajeevan N, Liu L, Zhao YO, Heisig J, Pan JY, et al. Gut Microbiota of the Tick Vector Ixodes scapularis Modulate Colonization of the Lyme Disease Spirochete. Cell Host Microbe. 2014;15(1):58–71. WOS:000330854100009. pmid:24439898 - 20.
Kato N, Mueller CR, Fuchs JF, Mcelroy K, Wessely V, Higgs S, et al. Evaluation of the Function of a Type I Peritrophic Matrix as a Physical Barrier for Midgut Epithelium Invasion by Mosquito-Borne Pathogens in Aedes aegypti. Vector-Borne Zoonot. 2008;8(5):701–712. WOS:000260062300014. pmid:18627241 - 21.
Talyuli OAC, Oliveira JHM, Bottino-Rojas V, Silveira GO, Alvarenga PH, Barletta ABF, et al. The Aedes aegypti peritrophic matrix controls arbovirus vector competence through HPx1, a heme-induced peroxidase. PLoS Pathog. 2023;19(2). ARTN e1011149 WOS:000967532500001. pmid:36780872 - 22.
Song XM, Wang MF, Dong L, Zhu HM, Wang JW. PGRP-LD mediates An. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis. PLoS Pathog. 2018;14(2). ARTN e1006899 WOS:000426477000040. pmid:29489896 - 23.
Weiss BL, Wang JW, Maltz MA, Wu YN, Aksoy S. Trypanosome Infection Establishment in the Tsetse Fly Gut Is Influenced by Microbiome-Regulated Host Immune Barriers. PLoS Pathog. 2013;9(4). ARTN e1003318 WOS:000318072700055. pmid:23637607 - 24.
Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2011;108(38):15966–15971. WOS:000295030000061. pmid:21896728 - 25.
Talaro KP, Chess B. Foundations in Microbiology. 9th ed. McGraw Hill; 2014. - 26.
Auer GK, Weibel DB. Bacterial Cell Mechanics. Biochemistry. 2017;56(29):3710–3724. pmid:28666084; PubMed Central PMCID: PMC6260806. - 27.
Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, et al. Monomeric and Polymeric Gram-Negative Peptidoglycan but Not Purified LPS Stimulate the Drosophila IMD Pathway. Immunity. 2004;20:637–649. - 28.
MacKenzie SA, Roher N, Boltaña S, Goetz FW. Peptidoglycan, not endotoxin, is the key mediator of cytokine gene expression induced in rainbow trout macrophages by crude LPS. Mol Immunol. 2010;47(7–8):1450–1457. pmid:20304498 - 29.
Chavshin AR, Oshaghi MA, Vatandoost H, Pourmand MR, Raeisi A, Terenius O. Isolation and identification of culturable bacteria from wild Anopheles culicifacies, a first step in a paratransgenesis approach. Parasit Vectors. 2014;7:419. pmid:25189316; PubMed Central PMCID: PMC4261757. - 30.
Devenport M, Fujioka H, Donnelly-Doman M, Shen Z, Jacobs-Lorena M. Storage and secretion of Ag-Aper14, a novel peritrophic matrix protein, and Ag-Muc1 from the mosquito Anopheles gambiae. Cell Tissue Res. 2005;320(1):175–185. pmid:15726420. - 31.
Shao L, Devenport M, Fujioka H, Ghosh A, Jacobs-Lorena M. Identification and characterization of a novel peritrophic matrix protein, Ae-Aper50, and the microvillar membrane protein, AEG12, from the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2005;35(9):947–959. pmid:15978997. - 32.
Devenport M, Fujioka H, Jacobs-Lorena M. Storage and secretion of the peritrophic matrix protein Ag-Aper1 and trypsin in the midgut of Anopheles gambiae. Insect Mol Biol. 2004;13(4):349–358. pmid:15271206. - 33.
Dinglasan RR, Devenport M, Florens L, Johnson JR, McHugh CA, Donnelly-Doman M, et al. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem Mol Biol. 2009;39(2):125–134. pmid:19038338; PubMed Central PMCID: PMC2684889. - 34.
Zhang GW, Niu GD, Franca CM, Dong YM, Wang XH, Butler NS, et al. Anopheles Midgut FREP1 Mediates Plasmodium Invasion. J Biol Chem. 2015;290(27):16490–16501. WOS:000357572800003. pmid:25991725 - 35.
Niu GD, França C, Zhang GW, Roobsoong W, Nguitragool W, Wang XH, et al. The fibrinogen-like domain of FREP1 protein is a broad-spectrum malaria transmission-blocking vaccine antigen. J Biol Chem. 2017;292(28):11960–11969. WOS:000405485600031. pmid:28533429 - 36.
Yassine H, Osta MA. Anopheles gambiae innate immunity. Cell Microbiol. 2010;12(1):1–9. pmid:19804484. - 37.
Mizutani T, Kobayashi M, Eshita Y, Inanami O, Yamamori T, Goto A, et al. Characterization of JNK-like protein derived from a mosquito cell line, C6/36. Insect Mol Biol. 2003;12(1):61–66. pmid:12542636. - 38.
Lin CC, Chou CM, Hsu YL, Lien JC, Wang YM, Chen ST, et al. Characterization of two mosquito STATs, AaSTAT and CtSTAT: Differential regulation of tyrosine phosphorylation and DNA binding activity by lipopolysaccharide treatment and by Japanese encephalitis virus infection. J Biol Chem. 2004;279(5):3308–3317. pmid:14607839. - 39.
Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7(7):715–723. WOS:000238377700016. pmid:16767093 - 40.
Meister S, Agianian B, Turlure F, Relogio A, Morlais I, Kafatos FC, et al. Anopheles gambiae PGRPLC-Mediated Defense against Bacteria Modulates Infections with Malaria Parasites. PLoS Pathog. 2009;5(8). ARTN e1000542 WOS:000270804500027. pmid:19662170 - 41.
Bahuguna S, Atilano M, Glittenberg M, Lee DH, Arora S, Wang LH, et al. Bacterial recognition by PGRP-SA and downstream signalling by Toll/DIF sustain commensal gut bacteria in Drosophila. PLoS Genet. 2022;18(1). ARTN e1009992 WOS:000936508700002. pmid:35007276 - 42.
Wang LH, Weber AN, Atilano ML, Filipe SR, Gay NJ, Ligoxygakis P. Sensing of Gram-positive bacteria in Drosophila: GNBP1 is needed to process and present peptidoglycan to PGRP-SA. EMBO J. 2006;25(20):5005–5014. WOS:000241989900029. pmid:17024181 - 43.
Meister S, Agianian B, Turlure F, Relógio A, Morlais I, Kafatos FC, et al. Anopheles gambiae PGRPLC-Mediated Defense against Bacteria Modulates Infections with Malaria Parasites. PLoS Pathog. 2009;5(8). ARTN e1000542 WOS:000270804500027. pmid:19662170 - 44.
Wang J, Song X, Wang M. Peptidoglycan recognition proteins in hematophagous arthropods. Dev Comp Immunol. 2018;83:89–95. pmid:29269264; PubMed Central PMCID: PMC5889321. - 45.
Wang S, Beerntsen BT. Functional implications of the peptidoglycan recognition proteins in the immunity of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2015;24(3):293–310. WOS:000353643000002. pmid:25588548 - 46.
Wang SJ, Beerntsen BT. Insights into the different functions of multiple peptidoglycan recognition proteins in the immune response against bacteria in the mosquito, Armigeres subalbatus. Insect Biochem Mol Biol. 2013;43(6):533–543. WOS:000319539100005. pmid:23541606 - 47.
Gabrieli P, Caccia S, Varotto-Boccazzi I, Arnoldi I, Barbieri G, Comandatore F, et al. Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission. Front Microbiol. 2021:12. ARTN 630438 WOS:000641273800001. pmid:33889137 - 48.
Wolf AJ, Underhill DM. Peptidoglycan recognition by the innate immune system. Nat Rev Immunol. 2018;18(4):243–254. pmid:29292393. - 49.
Kleino A, Ramia NF, Bozkurt G, Shen Y, Nailwal H, Huang J, et al. Peptidoglycan-Sensing Receptors Trigger the Formation of Functional Amyloids of the Adaptor Protein Imd to Initiate Drosophila NF-kappaB Signaling. Immunity. 2017;47(4):635–47 e6. pmid:29045898; PubMed Central PMCID: PMC5665175. - 50.
Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, et al. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol. 2003;4(5):478–484. WOS:000182665400016. pmid:12692550 - 51.
Kurz CL, Charroux B, Chaduli D, Viallat-Lieutaud A, Royet J. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. Elife. 2017;6. WOS:000397644100001. pmid:28264763 - 52.
Masuzzo A, Manière G, Viallat-Lieutaud A, Avazeri É, Zugasti O, Grosjean Y, et al. Peptidoglycan-dependent NF-κB activation in a small subset of brain octopaminergic neurons controls female oviposition. Elife. 2019;8. ARTN e50559 WOS:000518856300001. pmid:31661076 - 53.
Charroux B, Capo F, Kurz CL, Peslier S, Chaduli D, Viallat-lieutaud A, et al. Cytosolic and Secreted Peptidoglycan-Degrading Enzymes in Drosophila Respectively Control Local and Systemic Immune Responses to Microbiota. Cell Host Microbe. 2018;23(2):215–+. WOS:000425281900012. pmid:29398649 - 54.
Radhakrishnan P, Fedorka KM. Immune activation decreases sperm viability in both sexes and influences female sperm storage. P Roy Soc B-Biol Sci. 2012;279(1742):3577–3583. WOS:000306832100026. pmid:22696524 - 55.
Soldano A, Alpizar YA, Boonen B, Franco L, López-Requena A, Liu GD, et al. Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila. Elife. 2016;5. ARTN e13133 WOS:000378649800001. pmid:27296646 - 56.
Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A. 2010;107(18):8440–8445. WOS:000277310400071. pmid:20404155 - 57.
Yanagawa A, Couto A, Sandoz JC, Hata T, Mitra A, Agha MA, et al. LPS perception through taste-induced reflex in Drosophila melanogaster. J Insect Physiol. 2019;112:39–47. WOS:000456228600006. pmid:30528842 - 58.
Hillyer JF. Insect immunology and hematopoiesis. Dev Comp Immunol. 2016;58:102–118. WOS:000372764400011. pmid:26695127 - 59.
Shin SW, Kokoza V, Bian G, Cheon HM, Kim YJ, Raikhel AS. REL1, a Homologue of Drosophila Dorsal, Regulates Toll Antifungal Immune Pathway in the Female Mosquito Aedes aegypti. J Biol Chem. 2005;280(16):16499–16507. pmid:15722339. - 60.
Zakovic S, Levashina EA. NF-kappaB-Like Signaling Pathway REL2 in Immune Defenses of the Malaria Vector Anopheles gambiae. Front Cell Infect Microbiol. 2017;7:258. pmid:28680852; PubMed Central PMCID: PMC5478692. - 61.
Meister S, Kanzok SM, Zheng XL, Luna C, Li TR, Hoa NT, et al. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc Natl Acad Sci U S A. 2005;102(32):11420–11425. pmid:16076953; PubMed Central PMCID: PMC1183586. - 62.
Antonova Y, Alvarez KS, Kim YJ, Kokoza V, Raikhel AS. The role of NF-κB factor REL2 in the Aedes aegypti immune response. Insect Biochem Molec. 2009;39(4):303–314. WOS:000265305600008. pmid:19552893 - 63.
Myllymaki H, Valanne S, Ramet M. The Drosophila imd signaling pathway. J Immunol. 2014;192(8):3455–3462. WOS:000334312900001. pmid:24706930 - 64.
Rodgers FH, Cai JA, Pitaluga AN, Mengin-Lecreulx D, Gendrin M, Christophides GK. Functional analysis of the three major PGRPLC isoforms in the midgut of the malaria mosquito Anopheles coluzzii. Insect Biochem Molec. 2020:118. ARTN 103288 WOS:000518872600008. pmid:31760136 - 65.
Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong YM, et al. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathog. 2012;8(6). ARTN e1002737 WOS:000305987800011. pmid:22685401 - 66.
Carvalhoa L, Jacinto A, Matova N. The Toll/NF-kappa B signaling pathway is required for epidermal wound repair in Drosophila. Proc Natl Acad Sci U S A. 2014;111(50):E5373–E5382. WOS:000346366500009. pmid:25427801 - 67.
Khor S, Cai DS. Control of lifespan and survival by Drosophila NF-κB signaling through neuroendocrine cells and neuroblasts. Aging-Us. 2020;12(24):24604–22. WOS:000605608700013. - 68.
Nandy A, Lin L, Velentzas PD, Wu LP, Baehrecke EH, Silverman N. The NF-kappa B Factor Relish Regulates Atg1 Expression and Controls Autophagy. Cell Rep. 2018;25(8):2110. WOS:000450794200012. pmid:30463009 - 69.
Villalon JM, Ghosh A, Jacobs-Lorena M. The peritrophic matrix limits the rate of digestion in adult Anopheles stephensi and Aedes aegypti mosquitoes. J Insect Physiol. 2003;49(10):891–895. WOS:000185965800001. pmid:14511821 - 70.
Crampton JM, Beard CB, Louis C. The Molecular Biology of Insect Disease Vectors: A Methods Manual. Springer Dordrecht; 1997. - 71.
Benedict MQ. Care and maintenance of anopheline mosquito colonies. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors. Springer Netherlands; 1997. p. 3–12. - 72.
Holmes DS, Bonner J. Preparation, molecular-weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973;12(12):2330–2338. WOS:A1973P809300023. pmid:4710584 - 73.
Dong Y, Manfredini F, Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5(5):e1000423. pmid:19424427; PubMed Central PMCID: PMC2673032. - 74.
Popov SG, Villasmil R, Bernardi J, Grene E, Cardwell J, Popova T, et al. Effect of Bacillus anthracis lethal toxin on human peripheral blood mononuclear cells. FEBS Lett. 2002;527(1–3):211–215. pmid:12220662. - 75.
Yoneda M, Hirofuji T, Motooka N, Nozoe K, Shigenaga K, Anan H, et al. Humoral Immune Responses to S-Layer-Like Proteins of Bacteroides forsythus. Clin Diagn Lab Immunol. 2003;10(3):383–387. WOS:000183022900009. - 76.
Bick PH, Carpenter AB, Holdeman LV, Miller GA, Ranney RR, Palcanis KG, et al. Polyclonal B-Cell activation Induced by extracts of Gram-negative bacteria isolated from periodontally diseased sites. Infect Immun. 1981;34(1):43–49. WOS:A1981MJ13600009. pmid:6975240 - 77.
Bahia AC, Dong Y, Blumberg BJ, Mlambo G, Tripathi A, BenMarzouk-Hidalgo OJ, et al. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ Microbiol. 2014;16(9):2980–2994. pmid:24428613; PubMed Central PMCID: PMC4099322. - 78.
Kaneko T, Golenbock D, Silverman N. Peptidoglycan recognition by the Drosophila Imd pathway. J Endotoxin Res. 2005;11(6):383–389. WOS:000233794100009. pmid:16303095 - 79.
Dimopoulos G, Richman A, dellaTorre A, Kafatos FC, Louis C. Identification and characterization of differentially expressed cDNAs of the vector mosquito, Anopheles gambiae. Proc Natl Acad Sci U S A. 1996;93(23):13066–13071. WOS:A1996VT05400072. pmid:8917545 - 80.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. WOS:000173949500003 pmid:11846609 - 81.
Gao L, Song X, Wang J. Gut microbiota is essential in PGRP-LA regulated immune protection against Plasmodium berghei infection. Parasit Vectors. 2020;13(1):3. Epub 20200106. pmid:31907025; PubMed Central PMCID: PMC6945779. - 82.
Kowalczyk KM, Petersen J. Fission Yeast SCYL1/2 Homologue Ppk32: A Novel Regulator of TOR Signalling That Governs Survival during Brefeldin A Induced Stress to Protein Trafficking. PLoS Genet. 2016;12(5). ARTN e1006041 WOS:000377197100041. pmid:27191590
ADVERTISEMENT:
Hai, sobat pengemar slot! pernahkah mendengar semboyan “slot gacor”? Kalau belum, bersiaplah jatuh cinta sama program ini. raja slot merupakan mesin slots yang sering memberi win. Ya, mesin-mesin ini bisa disebut adalah andalannya tuk bawa come back cuan. tapi, gimana sih caranya jumpain slot demo yang benar? Santuy Bro and Sis, kita beri santai aja di sini
Gaming terpopuler waktu ini satu-satunya berada Indonesia yaitu akan memberikan ROI terbesar
SEGERA hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia