Abstract
Competitive bacteria like the human pathogen Pseudomonas aeruginosa can acquire iron from different iron carriers, which are usually internalized via outer membrane TonB-dependent receptors (TBDRs). Production of TBDRs is promoted by the presence of the substrate. This regulation often entails a signal transfer pathway known as cell-surface signaling (CSS) that involves the TBDR itself that also functions as transducer (and is thus referred to as TBDT), a cytoplasmic membrane-bound anti-σ factor, and an extracytoplasmic function σ (σECF) factor. TBDTs contain an extra N-terminal domain known as signaling domain (SD) required for the signal transfer activity of these receptors. In the current CSS model, presence of the signal allows the interaction between the TBDT and the anti-σ factor in the periplasm, promoting the proteolysis of the anti-σ factor and in turn the σECF-dependent transcription of response genes, including the TBDT gene. However, recent evidence shows that σECF activity does not depend on this interaction, suggesting that the contact between these 2 proteins fulfills a different role. Using the P. aeruginosa Fox CSS system as model, we show here that the SD of the FoxA TBDT already interacts with the C-terminal domain of the FoxR anti-σ factor in absence of the signal. This interaction protects FoxR from proteolysis in turn preventing transcription of σFoxI-dependent genes. By structural modeling of the FoxR/FoxASD interaction, we have identified the interaction sites between these 2 proteins and provide the molecular details of this interaction. We furthermore show that to exert this protective role, FoxA undergoes proteolytic cleavage, denoting a change in the paradigm of the current CSS model.
Citation: Wettstadt S, Marcos-Torres FJ, Otero-Asman JR, García-Puente A, Ortega Á, Llamas MA (2024) Bacterial TonB-dependent transducers interact with the anti-σ factor in absence of the inducing signal protecting it from proteolysis. PLoS Biol 22(12):
e3002920.
https://doi.org/10.1371/journal.pbio.3002920
Academic Editor: Ann M. Stock, Rutgers University-Robert Wood Johnson Medical School, UNITED STATES OF AMERICA
Received: July 26, 2024; Accepted: October 31, 2024; Published: December 2, 2024
Copyright: © 2024 Wettstadt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files. Raw data is available at the Mendeley Data repository (Mendeley Data, V1, doi:10.17632/nxh4c8ymnn.2).
Funding: This work was funded by MCIN/AEI/10.13039/501100011033 Spanish agency with projects BIO2017-83763-P and PID2020-115682GB-I00 to MLL. JOA was supported by the Spanish Ministry of Economy through an FPI fellowship (BES-2013-066301) and AO with grant PID2021‐122202OB‐I00. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist
Abbreviations:
ASD,
anti-σ domain; AUC,
analytical ultracentrifugation; CSS,
cell-surface signaling; CTD,
C-terminal domain; FPE,
filamentous phage extrusion; PAE,
predicted alignment error; PMF,
proton motive force; RIP,
regulated intramembrane proteolysis; RMSD,
root mean square deviation; SD,
signaling domain; STN,
secretin and TonB-dependent receptors N-domain; S1P,
site-1 protease; S2P,
site-2 protease; TBDR,
TonB-dependent receptor; TBDT,
TonB-dependent transducer; T2SS,
type 2 secretion system; T3SS,
type 3 secretion system; T4P,
type 4 pili system
Introduction
Iron is essential for life. However, despite being one of the most abundant elements on Earth, the bioavailability of iron is very limited because in our oxygen-rich atmosphere this metal readily oxidizes and precipitates in insoluble hydroxides. To acquire iron, bacteria produce and secrete high-affinity iron-scavenging compounds known as siderophores that keep iron in a chelated soluble state. Gram-negative bacteria take up iron-siderophore complexes via specific outer membrane receptors of the TonB-dependent receptor family (TBDR). TBDRs belong to the outer membrane β-barrel protein superfamily and share a common fold: a C-terminal 22-stranded antiparallel transmembrane β-barrel domain that forms a pore, and an N-terminal globular domain named plug, hatch, or cork (S1A Fig) [1]. The plug domain contains a mixed four-stranded β-sheet that is located inside the C-terminal barrel domain, occluding the pore in absence of the siderophore. Transport of the iron-siderophore complex requires energy in the form of proton motive force (PMF) and the TonB-ExbBD cytoplasmic membrane protein complex to transduce the energy to the outer membrane (S1B Fig) [1]. TonB is the protein of the complex that interacts with TBDRs in a 6-amino acids region located before the plug region termed the TonB box (S1A Fig).
Besides producing their own siderophores, most competitive bacteria use piracy strategies to acquire essential components like iron, thus increasing the capacity of the bacteria to thrive in many different environments. This is the case of the human pathogen Pseudomonas aeruginosa, which is very efficient in acquiring siderophores produced by other microorganisms (denoted xenosiderophores) and iron carriers from the host [2]. This capacity resides in the production of 34 different TBDRs, many of which are involved in the transport of iron-containing compounds [3,4]. Importantly, P. aeruginosa adjusts the production of TBDRs to the presence of their substrate. This regulation often entails a signal transduction circuit named cell-surface signaling (CSS) that involves the TBDR itself, a cytoplasmic membrane-bound anti-σ factor, and an extracytoplasmic function σ (σECF) factor in the cytosol (S1B Fig) [5–7]. Binding of the substrate to the receptor is transduced to the anti-σ factor in the periplasm resulting in the liberation of the σECF factor in the cytosol. The σECF factor then binds to the RNA polymerase (RNAP) and promotes the transcription of signal-response genes, including that of the receptor gene (S1B Fig), thus increasing the capacity of the bacteria to transport the substrate. TBDRs with this dual function in transport and signaling have been named TonB-dependent transducers (TBDTs) [8]. TBDTs contain an extra N-terminal domain known as signaling domain (SD) that is not present in TBDRs not involved in signaling. This domain is connected to the plug domain by a periplasmic flexible loop that includes the TonB box region (S1A Fig) [9,10]. The SD consists of 2 α-helices positioned side by side which are sandwiched between 2 β-sheets with the sheets made of 2 and 3 β-strands (S1A Fig) [9,11,12]. The structure of this domain resembles the periplasmic domain of outer membrane secretins of bacterial secretion systems, a domain that has been denoted STN (from secretin and TonB-dependent receptors N-domain; SMART accession number SM00965). STN domains interact with each other, with STN domains from other secretins, and with the periplasmic domains of cytoplasmic membrane proteins [13,14]. Similarly, the SD of a TBDT interacts with the periplasmic C-terminal domain of its cognate CSS anti-σ factor.
CSS anti-σ factors are cytoplasmic membrane-anchored proteins that contain 3 domains: a cytosolic N-terminal domain that binds the σECF factor blocking its interaction with the RNAP in absence of the CSS signal and is thus known as anti-σ domain (ASD), a single-pass transmembrane helix, and a periplasmic C-terminal domain (CTD) that receives the signal from the TBDT [5–7]. The crystal structure of the ASD of the PupR CSS anti-σ factor showed that it folds into a three-helix bundle and forms a dimer in solution [15]. PupR seems to lack an ordered fourth helix found in cytosolic anti-σ factors like ChrR or RslA. This helix serves to block the interaction of the σECF factor with the RNAP. Therefore, it has been proposed that CSS anti-σ factors prevent the σECF factor/RNAP interaction by sandwiching the σECF factor between the ASD dimer thus tethering the σECF factor to the membrane and limiting access for the RNAP [15]. The structure of the CTD of PupR has also been solved and revealed that this domain can be divided into 2 subdomains; an N-terminal subdomain close to the cytoplasmic membrane, and a C-terminal subdomain that shows high structural similarity to the STN domain of secretins and the SD of TBDTs [16].
In the current CSS model, signal perception promotes the interaction between the SD and the anti-σ factor in the periplasm. This model is mainly based on earlier results obtained with the Escherichia coli Fec system. By introducing mutations that compromise the interaction between the TBDT FecA and the anti-σ factor FecR, σFecI activity was reduced in response to the inducing signal ferric citrate [17,18]. However, our more recent results have shown that the SD/anti-σ factor interaction can be abolished without inhibiting σECF activity [19], which suggests that TBDTs could function in a different way than initially thought. Importantly, signal perception does not induce alterations in the overall structure of the SD and only modifies the position of this domain [10,12,20]. The region that connects the SD to the plug and β-barrel is long and flexible and has been proposed to enable movement of the SD in the periplasm upon signal perception, thereby promoting the interaction with the anti-σ factor. However, although the position of the SD with respect to the β-barrel in the outer membrane indeed changes in response to the inducing signal, the SD does not extend further into the periplasm [10,20]. This raises the question of how the signal is transmitted from the outer membrane to the cytosol to modify the activity of the CSS σECF factor. Our previous work using the P. aeruginosa Fox CSS system as a model has shed light on this matter by showing that signal transmission entails the regulated proteolysis of the anti-σ factor FoxR (S1B Fig) [21–23]. The TBDT of this system, the FoxA protein, recognizes and imports the xenosiderophore ferrioxamine B, among other structurally related compounds [24–27]. Besides being processed in presence of the signal, FoxR undergoes self-cleavage prior to signal recognition by an NO-acyl shift between glycine-191 and threonine-192 (GT site) [22,28]. This process separates the protein into 2 domains (FoxRN and FoxRC, respectively) that are both required for proper signal transduction in response to ferrioxamine [22]. In our model (S1B Fig), presence of ferrioxamine B leads to the degradation of FoxRC in a process that involves the proteolytic activity of the C-terminal processing periplasmic proteases Prc and CtpA [23]. This allows the regulated intramembrane proteolysis (RIP) of the FoxRN domain by a still unknown site-1 protease (S1P) and the RseP site-2 protease (S2P) [21–23]. It is at present unclear how the proteolysis of FoxR is initiated in response to ferrioxamine B. One possibility is that the proteases are activated in response to the signal. However, CSS anti-σ factors lacking part of the C-terminal domain can be processed in absence of the inducing signal [19], which indicates that the proteases are active and functional in non-inducing conditions. This is in accordance with Prc, CtpA, and RseP being required for several other processes in the cell not related to iron acquisition or anti-σ factor proteolysis. Therefore, we hypothesize that FoxR is protected from proteolysis in the absence of ferrioxamine while presence of the siderophore exposes the protease-sensitive domains of the protein, and that the signaling domain of FoxA (FoxASD) plays a role in this process. To get more insights into the CSS mechanism, we have analyzed in this work the interaction between the SD of the P. aeruginosa FoxA TBDT and the CTD of the FoxR anti-σ factor and its effect on σFoxI-mediated transcription both in vivo and in vitro. We show that overproduction of the SD of several Pseudomonas TBDTs blocks signaling and proteolysis of their cognate anti-σ factor, and that co-expression of FoxR with FoxASD stabilizes the anti-σ factor. Moreover, we have determined that FoxRC is the domain that interacts with FoxASD and we provide the molecular details of this interaction. By structural modeling of the FoxR/FoxASD interaction, we have identified the interaction sites between these 2 domains. Finally, we show that FoxASD is cleaved in vivo likely by the periplasmic protease CtpA. Based on these results, we propose a new model for CSS in which the SD of the TBDT and the anti-σ factor interact in the absence of the inducing signal that prevents the proteolysis of the anti-σ factor and thus blocks the activation of the CSS pathway.
Results
Overproducing the signaling domain (SD) of TBDTs inhibits CSS activity
To assess the effect of the SD of the TBDT on CSS activity, the SD of several P. aeruginosa (Pa) and Pseudomonas putida (Pp) TBDTs were overproduced from plasmid. All constructs used contain the signal sequence of the corresponding protein (see S1 Table) to allow export of the SD into the periplasm. CSS activity was analyzed using CSS-dependent transcriptional fusions in which the promoter region of the TBDT gene, which is one of the genes transcribed by the σECF factor in response to the CSS signal (S1B Fig) [5,6], was placed in front of the lacZ gene. By measuring β-galactosidase activity, we followed the activation of the CSS signaling pathway, which involves the release of the σECF factor and transcription from the TBDT gene promoter. We analyzed the activities of the Fox, Fiu, and Iut CSS systems, which respond to the xenosiderophores ferrioxamine, ferrichrome, and aerobactin, respectively, and that of the P. aeruginosa Hxu system that responds to the presence of haem in the extracellular medium [21,29]. Overproducing each of the SDs completely inhibited CSS activities and resulted in bacteria unable to respond to the corresponding CSS-inducing signal (Fig 1A).
Fig 1. Effect of overproducing the SDs of CSS receptors on CSS activity and anti-σ factor proteolysis.
In both panels, P. putida KT2440 or the P. aeruginosa PAO1 wild-type strains bear the empty pBBR1MCS-5 plasmid (-) or the pBBR1MCS-5-derived plasmid expressing the indicated SD (+ Pp- or Pa-SD) (S1 Table). Strains were grown in iron-restricted conditions (- signal) or iron-restricted medium supplemented with the cognate CSS inducing signal (+ signal), i.e., 1 μm ferrioxamine B for the Pp and Pa Fox systems, 40 μm ferrichrome for the Pp and Pa Fiu systems, aerobactin-containing supernatant for the Pp Iut system, and 20 μm haem for the Pa Hxu system. 1 mM IPTG was added to the cultures used in panel B. (A) β-galactosidase activity of the indicated lacZ fusion genes produced from pMP220-derived plasmids (S1 Table). Data are means ± SD from 3 biological replicates (N = 3). P-values were calculated by two-tailed t test by comparing the value obtained in the strain overexpressing the SD with that of the wild-type strain in the same growth condition and are represented in the graphs by ***, P P (B) Western-blot analyses of P. aeruginosa HA-FoxR anti-σ factor and P. putida HA-IutY σ/anti-σ factor hybrid protein produced from pMMB67EH-derived plasmids (S1 Table). Proteins were immunoblotted against the HA-epitope using a monoclonal antibody. Positions of the protein fragments and the molecular size marker (in kDa) are indicated. Presence of the HA-tag adds ∼1 kDa to the molar mass of the protein fragments. Blots are representatives of at least 3 biological replicates (N = 3). The raw data underlying the graphs shown in the figure can be found at Mendeley Data repository (Mendeley Data, V1, 10.17632/nxh4c8ymnn.2). Western blot can be found in S1 Raw Images. CSS, cell-surface signaling; SD, signaling domain.
Then, we performed western blot analysis of the anti-σ factor component of the P. aeruginosa Fox and the P. putida Iut CSS systems (the FoxR and the IutY proteins, respectively) using N-terminally HA-tagged proteins produced from plasmid. These analyses showed that overproducing the SD of the cognate TBDT (FoxA and IutA, respectively) inhibits the RIP of these proteins in response to the inducing signal (Fig 1B). This is in agreement with the bacteria being unable to respond to the siderophore (Fig 1A) because blocking the RIP mechanism is known to prevent the liberation and activation of the σECF factor (S1B Fig) [6,21]. Together, these results indicate that the SD of the TBDT can protect the anti-σ factor from RIP.
The signaling domain of FoxA stabilizes FoxR in vivo
To further understand the function of the SD of the receptor in the CSS pathway, we analyzed the stability of the P. aeruginosa FoxR anti-σ factor in strains overproducing FoxASD by western blot using a polyclonal anti-FoxR antibody (S2 Fig). Because the antibody was not able to detect the chromosomically produced FoxR protein (S3A Fig), we aimed to detect FoxR proteins expressed constitutively from a Ptac promoter on the pMMB67EH plasmid. Three different FoxR proteins were co-expressed with FoxASD in a P. aeruginosa ΔfoxR mutant: the wild-type protein, and N- and C-terminal HA-tagged proteins, which we have used in previous studies to detect FoxR using an anti-HA antibody [22,28] (S2B and S2C Fig, FoxR, HA-FoxR, and FoxR-HA, respectively). Importantly, the 3 proteins were able to complement the phenotype of a ΔfoxR mutant (S3B Fig), indicating that they are functional. Strains were grown in iron-restricted medium supplemented with ferrioxamine and proteins were immunoblotted using polyclonal antibodies raised against the periplasmic domain of FoxR (S2B Fig, upper panel) or the SD of FoxA (S2B Fig, middle panel). Samples were also immunoblotted against the OprL protein as a loading control (S2B Fig, lower panel). In the strain expressing the non-tagged FoxR protein, protein bands were not detectable when FoxASD was absent (S2B Fig, lane 2). When the 2 proteins were co-expressed, 3 FoxR protein bands were detected (S2B Fig, lane 3), corresponding to the FoxR full-length protein (approximately 36 kDa), and the 2 protein domains that are formed upon the spontaneous cleavage of FoxR, FoxRN (approximately 21 kDa), and FoxRC (approximately 15 kDa) (S1B and S2A Figs). Producing the HA-tagged proteins resulted in FoxR bands of higher intensity, and protein bands were detected also in strains not producing FoxASD (S2B Fig, upper panel). However, as occurred with the non-tagged protein, co-expression of the FoxR HA-tagged proteins with FoxASD considerably increased the intensity of the FoxR protein bands (S2B Fig, lanes 5 and 7). The same 3 protein fragments detected with FoxR were detected with the HA-tagged versions (note that the HA epitope adds 1 kDa to the molecular weight of the corresponding protein band) (S2B Fig, upper panel). In addition, an extra band of approximately 32 to 33 kDa was detected with FoxR-HA (S2B Fig, lanes 6–7). This FoxR truncate is likely a consequence of the presence of the HA epitope in the C-terminal end because this band was not detected in the non-tagged or the N-terminally HA-tagged versions. Altogether, these results show that FoxASD can stabilize FoxR preventing it from degradation. Importantly, this effect depends on the presence of ferrioxamine because it does not occur when the strains are grown in iron-sufficient conditions (S2C Fig). In this condition, the intensities of all FoxR protein bands were higher than the intensities of the FoxR protein bands when bacteria were grown in the presence of ferrioxamine and did not increase when FoxR was co-produced with FoxASD (S2C Fig). This suggests that under iron limitation and in the presence of ferrioxamine, a cell factor destabilizes FoxR probably by proteolysis. Therefore, overproducing FoxASD impedes both the regulated proteolysis of FoxR (Fig 1B) and its degradation (S2B Fig) thus blocking CSS activity (Fig 1A).
The C-terminal domain of FoxR interacts with the signaling domain of FoxA
The experiments performed above suggest that FoxASD interacts with FoxR protecting it from proteolysis. To analyze this interaction, we performed two-hybrid experiments using the bacterial adenylate cyclase-based two-hybrid (BACTH) system [30,31]. This system exploits the fact that the catalytic domain of adenylate cyclase (CyaA) from Bordetella pertussis consists of 2 complementary fragments, T25 and T18, that are not active when physically separated. When these 2 fragments are fused via 2 interacting polypeptides, the CyaA enzyme is functional and synthesizes cAMP, which is required to express the lactose (lac) operon (among other genes). The amount of cAMP produced by reconstituted CyaA can be thus measured by β-galactosidase assay using an E. coli cyaA negative strain [31]. Hence, we fused FoxASD (amino acids 1–89 of the FoxA mature protein) to the T25 fragment of the B. pertussis CyaA enzyme (FoxASD-T25) and different FoxR domains to the T18 fragment. We included the whole periplasmic domain of FoxR containing the T192A mutation that prevents the spontaneous cleavage of this protein [22] (FoxRperi-T192A, amino acids 107–328), and the FoxRC (amino acids 192–328) and FoxRN (amino acids 1–191) domains produced upon the spontaneous cleavage of this protein (Fig 2A). In addition, we included a FoxR fragment containing only the periplasmic part of FoxRN without the cytosolic and TM domains (FoxRperi-N, amino acids 107–191, Fig 2A). These constructs were introduced into the E. coli cyaA negative BTH101 strain bearing the FoxASD-T25 construct. As a negative control, we used a strain bearing the pKT25 and pUT18C empty plasmids (-), and as a positive control, a strain encoding T25 and T18 fragments fused to the GCN4 leucine-zipper dimerization motif (T25-zip and T18-zip) that produces a characteristic Cya+ phenotype [30] (Fig 2B). β-galactosidase assays showed that the Cya+ phenotype was restored when FoxASD was co-produced with the FoxRperi-T192A and the FoxRC domains but not with FoxRN or FoxRperi-N domains (Fig 2B). This indicates that FoxASD interacts with the C-domain of FoxR but not with its N-domain.
Fig 2. Analysis of the FoxR/FoxASD interaction in vivo by two-hybrid and in vitro by analytical ultracentrifugation.
(A) Schematic representation of the FoxR protein and the protein fragments used in the two-hybrid assay. The amino acids contained in each fragment are indicated in brackets. (B) Bacterial two-hybrid assay to test interactions between FoxASD and the different FoxR fragments shown in A. E. coli BTH101 bearing the pUTC18C and pKNT25 empty plasmids (- /-) or its derivatives encoding the indicated FoxR and FoxASD proteins were grown in LB with 0.5 mM IPTG. A strain bearing the pUTC18C-zip and pKNT25-zip plasmids (zip / zip) was used as a positive control. β-galactosidase assay was performed, and data are means ± SD from at least 3 biological replicates (N = 3). P-values were calculated by two-tailed t test by comparing the value obtained for one strain with that of the negative control (- / -) and are represented in the graphs by ***, P P (C) Biophysical characterization of the FoxR/FoxASD interaction. The upper panel shows the sedimentation velocity AUC analysis of FoxRperi, FoxASD, and the FoxRperi/FoxASD complex. Values given correspond to experiments conducted at 8°C in Tris 20 mM (pH 8.3), NaCl 500 mM, DTT 1 mM, Glycerol 3% buffer and have not been standardized to s20,w. The lower panel shows the sw isotherm analysis of the sedimentation coefficients from a FoxRperi/FoxASD titration. The solid curve indicates the best fit of a global analysis of 2 sw-isotherms (absorbance and interference data). The bottom panel shows the residual plot. The raw data underlying the graphs shown in the figure can be found at Mendeley Data repository (Mendeley Data, V1, 10.17632/nxh4c8ymnn.2). AUC, analytical ultracentrifugation;
To investigate the oligomeric states of FoxRperi and FoxASD protein domains and the stoichiometry of the FoxRperi/FoxASD complex, we performed sedimentation velocity analytical ultracentrifugation (AUC). FoxASD and 3 different FoxR domains (FoxRperi-N, FoxRC, and FoxRperi-T192A, Fig 2A) were N-terminally tagged with a 6xHis epitope and heterologously produced in E. coli for protein purification. Analyses of the expression and solubility of the Fox domains showed that FoxRC and FoxRperi-N were highly insoluble (S4 Fig). However, FoxRperi-T192A containing the whole periplasmic region of FoxR was soluble, as was FoxASD (S4 Fig). Thus, these 2 protein domains were purified by FPLC and used in the AUC experiments. The theoretical standardized sedimentation values (s20,w, indicating values at 20°C and in water solvent) were estimated by hydrodynamic modeling using HYDROPRO [32] on the atomic detailed structures of the monomeric proteins obtained from the AlphaFold2 database [33]. For FoxRperi the s20,w was estimated at 2.4 S and for FoxASD at 1.2 S. AUC was performed first with the single protein domains, both at a concentration of 15 μm. The obtained sedimentation coefficient profiles showed an almost unique sedimenting species for both proteins under the conditions assayed (Fig 2C, upper panel). Upon standardization of the values obtained to water and 20°C conditions (see Materials and methods), the sedimentation coefficients of these species were 2.1 S for FoxRperi and 1.2 S for FoxASD. These values correspond to the theoretically calculated coefficients of the monomers indicating that none of the protein domains oligomerized at the concentration assayed. The assay was then performed with FoxRperi and FoxASD in the same cell to evaluate their oligomerization states and the stoichiometry of the complex. Several concentration ratios were assayed (FoxRperi:FoxASD at 7.5 μm:15 μm, 15 μm:15 μm, and 15 μm:30 μm), showing all a similar sedimentation coefficient profile. In all cases, the sedimenting species corresponding to FoxASD was present while the species corresponding to FoxRperi disappeared (Fig 2C, upper panel). Instead, a new species with a s20,w at 2.9 S appeared (Fig 2C, upper panel). This value correlates with the theoretical sedimentation coefficient of the FoxRperi/FoxASD complex (2.7 S) calculated form the hydrodynamic model generated with AlphaFold2 (Fig 3A). This indicates that FoxRperi and FoxASD form a heterodimer complex. Finally, a series of protein concentration ratios were assayed by sedimentation velocity to estimate the binding parameters of the hetero association process. In these assays FoxRperi was kept fixed at 10 μm, and titrated with FoxASD at 1, 2, 5, 10, 20, 40, and 80 μm. The sedimentation coefficient profile obtained was then globally analyzed, extracting the weight average s for each profile of all the peaks corresponding to monomers and complex (it is a fast reaction boundary, rapid interacting system). An isotherm was generated from this data (Fig 2C, lower panel) and globally fitted through SEDPHAT [34] to an A+B AB model with a Kd = 4.3 μm. The sedimentation coefficient of the larger species is consistent with the 1:1 FoxRperi/FoxASD complex.
Fig 3. Structural prediction of the FoxR/FoxA interaction and effect of key residues on FoxR/FoxA interaction.
(A) The signaling domain of FoxA (in orange) interacts with FoxR (in blue) via their STN-like domains. Close-up view involving the residues predicted to participate in the FoxR/FoxA interaction and the distances between the interacting residues are shown. The interaction model was generated with Alphafold2 using the FoxRperi domain (amino acids 107–328, Fig 2A) and FoxA1-516. (B, C) Bacterial two-hybrid assay to test interactions of the 2 subdomains of FoxRC with FoxASD (B), and between the FoxRC and FoxASD protein variants bearing the indicated single residue change (C). E. coli BTH101 bearing the pUTC18C and pKNT25 empty plasmids (- /-) or its derivatives encoding the indicated FoxRC and FoxASD proteins were grown in LB with 0.5 mM IPTG. A strain bearing the pUTC18C-zip and pKNT25-zip plasmids (zip / zip) was used as a positive control. β-galactosidase assay was performed, and data are means ± SD from 3 (N = 3) biological replicates. P-values were calculated by two-tailed t test by comparing the value obtained in the strain bearing the changed protein variant(s) with that of the strain bearing the wild-type FoxRC / FoxASD constructs and are represented in the graphs by ns, no significant; **, P P P 10.17632/nxh4c8ymnn.2).
Structural prediction of the FoxR/FoxASD interaction
Next, we aimed at determining the structure of the FoxR protein and the FoxR/FoxASD complex. Therefore, highly purified FoxRperi-T192A and FoxASD protein domains were obtained by SEC-FPLC. However, several attempts to obtain crystals of the FoxR protein alone and the FoxR/FoxASD complex were unsuccessful. Therefore, we obtained structural predictions for these proteins. The structural model of the full-length FoxR protein was obtained from the Alphafold structure database (AF_AFQ9I115F1). As predicted from the amino acid sequence, FoxR has an N-terminal ASD spanning residues 1–80 that display an interlocked three-helix bundle followed by a flexible loop that connects it to the transmembrane region (residues 87–106) (S5A Fig). Two structural subdomains conforming the periplasmic portion of the protein are evident in the structural model (S5A Fig). The N-terminal subdomain spans residues 107–247 and is composed of 14 anti-parallel β-strands forming a twisted β-solenoid-like motif, while the C-terminal subdomain (residues 248–328) folds into an STN-like domain (STND) (S5A Fig). The structure of the periplasmic domain of FoxR is nearly identical to that of the crystal structure of anti-σ factor PupR (6OVM), with a root mean square deviation (RMSD) of 0.867 Å (S5B Fig). The most significant dissimilarities are in the 2 anti-parallel β strands at the beginning of the STND (S5C Fig).
As mentioned before, the C-terminal subdomain of the periplasmic region of FoxR (FoxRSTND) is a structural homologue of the FoxASD (PBD access code 6I97). Even though both domains share the same fold, they superimpose with an RMSD of 1.709 Å, denoting a significant number of structural differences (S6C Fig). Both domains share the same structural elements, although FoxRSTND presents an extra 5 amino acid α helix at the N-terminus (α1), followed by a common structure composed of 6 β strands and 3 α helices (α2, α3, and α4). Strands β1 and β3 form antiparallel β sheets at the N-terminus, the remaining β strands β2, β5, and β6 form C-terminal β sheets (S6A Fig). While lacking the first α helix, FoxASD is similarly composed of an N-terminal β sheet (β1’ and β3’), 3 α helices (α1’, α2’, and α3’), and the C-terminal β sheet (β3’, β4’, and β5’) (S6B Fig).
The interaction model between FoxRperi and FoxA we generated with Alphafold shows that both proteins are likely to interact through their STN domains with a predicted alignment error (PAE) 3A and S7). This agrees with the oligomeric nature of STN domains [13,14]. According to this prediction, the FoxR/FoxASD complex forms a 5-stranded β sheet composed of β1 and β3 from FoxRSTND, and β2’, β5’, and β6’ from FoxASD. This β sheet interface is created by 4 hydrogen bonds between the backbones of the β3 residues V294 and S292 from FoxRSTND and L80 and A82 in β2’ from FoxA (Fig 3A). This complex is further stabilized by 3 hydrogen bonds connecting the side chains of FoxA-S81 with residues Y295 and T310 of FoxR, which may be important for the specificity between both proteins since this is not a conserved residue in other TBDT proteins. Two salt bridges linking FoxR-D249 and -D255 to FoxA-R74, as well as 3 hydrogen bonds between FoxR residues T260, D249, and R296, and FoxA residues N70, R74, and G77, also seem to contribute to stabilizing the protein complex (Fig 3A). Many of these interactions are mirroring those observed for the crystal structure for the PupB/PupR complex [16], which supports the prediction of the interaction model (S7A Fig). In this complex, the backbone interactions between PupR-T288 and -S286 and PupB-L75 and -T77 shape the β sheet interface and the side chain of PupB-S76 stabilizes the interaction via 2 hydrogen bonds with PupR-A300 and -T304.
FoxRSTND is required for interactions with FoxASD
The structural modeling data suggested that FoxRSTND is the subdomain involved in interacting with FoxASD (Fig 3A). To analyze the importance of this subdomain of FoxR in the FoxR/FoxASD interaction, we performed BACHT 2 hybrid analyses using a FoxRC fragment lacking the STND subdomain and a fragment containing only this region of FoxR (FoxR192-255 and FoxRSTND amino acids 256–328, respectively, Fig 2A). As shown before, β-galactosidase activity increased when FoxASD was co-expressed with the whole FoxRC domain (residues 192–328) (Fig 3B). Activity also increased when FoxASD was co-expressed with FoxRSTND, but it did not when it was co-expressed with FoxR192-255 (Fig 3B). This indicates that FoxR192-255 does not interact with FoxASD and thus that the interaction between FoxR and FoxASD requires the C-terminal subdomain of FoxR homologous to FoxASD. This agrees with the structural prediction showing that the STND subdomain of FoxR interacts with FoxASD by forming a 5-stranded β sheet stabilized by α helices.
Residues FoxR-S292 and -G293, and FoxA-S81 are required for FoxRC/FoxASD interaction and CSS signaling
Because the Alphafold model of the FoxR/FoxA complex indicates that the region comprising residues S292 to V294 of FoxRSTND is required to create a β sheet interface, and the residue S81 of FoxA is required to stabilize this interface (Fig 3A), we decided to change these protein residues and analyze the FoxR/FoxA interaction by two-hybrid experiments. We selected the FoxRC-S292 and -G293, and FoxASD-S81 residues, and changed them to alanine (A) and proline (P). The FoxR-S292P change is expected to distort the β3 strand required to interact with FoxA, while the S292A change should not introduce significant structural alterations. Besides, due to the structural features of glycines, changing the FoxR-G293 residue may alter the proper formation of the β3 strand, also perturbing its interaction with FoxA. As for FoxA, a S81A change will impair the hydrogen bond interactions observed with FoxR-Y295 and -T310 residues without perturbing the overall structure of the β2’ strand, while a S81P change will impair both the hydrogen bonds and the β2’ strand formation. Our two-hybrid analyses using these constructs showed that none of the β3-distorting variants of FoxR were able to interact with FoxASD (Fig 3C). Similarly, the β2’-distorting FoxASD-S81P variant was not able to interact with FoxRC. However, the 2 variants that did not distort the secondary β structure of the proteins, the FoxASD-S81A and FoxRC-S292A variants, respectively, were able to interact with their partners to almost wild-type levels (Fig 3C). In fact, for FoxASD-S81A, we did not observe any significant decrease in binding despite the loss of the 3 hydrogen bonds in the interaction with FoxRC (Fig 3A). However, the interaction between FoxASD-S81A and FoxRC-S292A seems to be stronger than the interaction between wild-type FoxRC and FoxASD (Fig 3C) hinting at a more promiscuous binding towards alternative variants of FoxRC. Altogether, this suggests that the 3 hydrogen bonds coordinated by FoxA-S81 might be involved in the stabilization and/or specificity of the complex between both proteins, rather than being essential for complex formation.
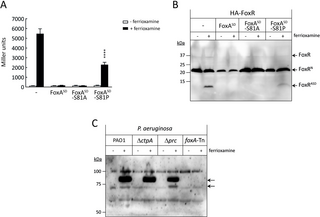
Impairing the FoxR/FoxA interaction may impact the proteolytic cascade and CSS activity. To analyze this effect, we overproduced the FoxASD-S81A and -S81P variants in the P. aeruginosa PAO1 wild-type strain and analyzed σFoxI activity (Fig 4A) and FoxR proteolysis (Fig 4B). Overexpression of the FoxASD-S81A variant blocks CSS activation and FoxR proteolysis in response to ferrioxamine, as also observed with the FoxASD wild-type (Fig 4A and 4B). In contrast, overproduction of the FoxASD-S81P variant reduced but did not block σFoxI activity and FoxR proteolysis (Fig 4A and 4B). Because FoxASD-S81A is still able to interact with FoxR while FoxASD-S81P is not (Fig 3C), these results indicate that the ability of FoxASD to protect FoxR from proteolysis and block CSS activity depends on its capacity to interact with the anti-σ factor.
Fig 4. Role of FoxASD in CSS activity.
(A) β-galactosidase activity of the P. aeruginosa foxA::lacZ fusion gene in the PAO1 wild-type bearing the empty pBBR1MCS-5 plasmid (-) or the pBBR1MCS-5-derived plasmid expressing the wild-type FoxASD protein or the indicated protein variant (S1 Table). Strains were grown in iron-restricted conditions without or with 1 μm ferrioxamine B. Data are means ± SD from 5 (N = 5) biological replicates. P-values were calculated by two-tailed t test by comparing the value obtained in the strain overproducing the single change residue protein variant with that of the wild-type FoxASD protein in the same growth condition and are represented in the graphs by ****, P (B) Western blot analyses of P. aeruginosa PAO1 producing an N-terminally HA-tagged FoxR anti-σ factor from a pMMB67EH-derived plasmid (S1 Table). Strains also bear the pBBR1MCS-5 empty plasmid (-) or the pBBR1MCS-5-derived plasmid expressing the wild-type FoxASD protein or the indicated protein variants (S1 Table) and were grown in iron-restricted conditions without (-) or with (+) 1 μm ferrioxamine B and 1 mM IPTG. Proteins were immunoblotted against the HA-epitope using a monoclonal antibody. Positions of the protein fragments and the molecular size marker (in kDa) are indicated. Presence of the HA-tag adds ∼1 kDa to the molar mass of the protein fragments. Blots are representatives of 3 biological replicates (N = 3). (C) P. aeruginosa PAO1 wild-type strain and the indicated isogenic mutants were grown to late log-phase under iron-restricted conditions and in the absence (-) or presence (+) of 1 μm ferrioxamine. Total proteins were prepared and immunoblotted against the FoxA TBDT using a polyclonal antibody directed against a peptide of the β-barrel of the protein. Positions of the protein fragments and the molecular size marker (in kDa) are indicated. Blot is a representative of 4 biological replicates (N = 4). The raw data underlying the graphs shown in the figure can be found at Mendeley Data repository (Mendeley Data, V1, 10.17632/nxh4c8ymnn.2). Western blot can be found in S1 Raw Images. CSS, cell-surface signaling; TBDT, TonB-dependent transducer.
The signaling domain of FoxA (FoxASD) is cleaved
Our earlier results indicate that the periplasmic proteases Prc and CtpA are required for proper CSS activation. While lack of Prc considerably reduces CSS activity in response to the inducing signal, lack of CtpA significantly increases CSS activity [23]. Absence of both proteases influence the proteolysis of the anti-σ factor FoxR but in opposite ways. While in a Δprc mutant the FoxRC domain is more stable, in accordance with Prc degrading this domain, in a ΔctpA mutant FoxRC is hardly detectable [23]. These phenotypes may be related to FoxA, as we have determined in this study that this receptor protects FoxR from degradation. Therefore, we decided to investigate the effect that the Prc and CtpA proteases have on FoxA. Stability of FoxA was analyzed by western blot in the PAO1 wild-type strain and the ΔctpA and Δprc mutants using a polyclonal antibody generated against a peptide of the β-barrel of the receptor (residues 331–344). A mutant unable to produce FoxA (foxA-Tn) was used as negative control. As expected, the FoxA receptor was not detected when ferrioxamine was not present in the medium while it was highly induced when the siderophore was added (Fig 4C). In this condition, a single protein band was observed in the PAO1 and ΔctpA mutants; however, 2 protein bands were obtained in the Δprc mutant (Fig 4C). Interestingly, the sizes of these protein bands resemble the size of the complete FoxA protein (~84 to 85 kDa) and of the FoxA protein without the signaling domain (~73 to 74 kDa). This suggest that not only FoxR but also FoxA is processed in vivo in the periplasm and that this event is required for proper CSS activity.
Discussion
In this study, we shed light on the molecular details of the signal transduction by CSS by analyzing the interaction between the TBDT and the anti-σ factor. We show that overproducing the SD of different TBDTs blocks the activation of the CSS pathway (Fig 1A) by preventing the proteolysis of the CSS anti-σ factor (Fig 1B) and thus the release of the σECF factor. This indicates that overproducing the SD of the receptor makes the anti-σ factor inaccessible to the proteases required to degrade and cleave this protein. In accordance with this, we show that the FoxR anti-σ factor is considerably more stable upon overproduction of FoxASD (S2 Fig). In fact, when purifying FoxRperi for the in vitro analyses, we observed that a high fraction of the protein precipitated upon dialysis into low-salt buffers or when concentrating it, while no precipitation was observed when the protein was dialyzed in presence of FoxASD. This further confirms that FoxA stabilizes the periplasmic domain of FoxR, as also observed with the PupB TBDT and the PupR anti-σ factor [16]. Based on these results, we hypothesized that in vivo, FoxASD interacts with FoxR in the absence of ferrioxamine, and that this interaction stabilizes and blocks the proteolysis of the anti-σ factor.
Three forms of the FoxR protein are detected in P. aeruginosa, the full-length protein (~36 kDa), the FoxRN domain (~21 kDa), and the FoxRC domain (~15 kDa) (S2 Fig). FoxRN and FoxRC are the result of the non-enzymatic self-cleavage of FoxR, which occurs prior to signal recognition by an NO-acyl shift between GT residues located within the periplasmic domain of FoxR [22,28]. We showed earlier that FoxRN and FoxRC interact with each other and function together in the signal transduction pathway [22]. FoxRC, but not FoxRN, is able to sense and generate the response to ferrioxamine, suggesting an interaction between FoxRC and FoxA [22]. The two-hybrid analyses performed in this work have demonstrated the interaction of FoxASD with FoxRC but not with FoxRN (Fig 2B). Specifically, FoxASD interacts with the subdomain comprising residues 256–328 of FoxRC, as shown by both the two-hybrid assay and the FoxR/FoxA predicted structure (Fig 3B and 3A). This domain is a structural homologue of the periplasmic domain of secretins and the signaling domain of TBDTs (the SNT domain). Secretins form megadalton bacterial membrane channels by assembling 12–15 monomers. Bacteria use these channels to transport large molecules across the outer membrane, including effectors of the type 2 secretion system (T2SS), filament accommodation of the type 3 secretion and type 4 (pili) systems (T3SS and T4P), or phage release in the filamentous phage extrusion (FPE) system [13,14]. Secretins contain a C-terminal domain that forms the channel in the outer membrane and a variable number of periplasmic STN-like domains connected via short linkers. The STNDs of one secretin monomer interact with both each other and the STNDs from other secretin molecules forming oligomers. Due to the similarities with secretin domains, one might hypothesize that the SD of TBDTs and the SNT domain of anti-σ factors form similar complexes in vivo. In this regard, it is noteworthy to mention that when FoxASD was overproduced, we detected protein bands of higher molecular weight than that of the FoxASD monomer (9 to 10 kDa) (S2B and S2C Fig, middle panel). These protein bands could be the result of FoxASD forming dimers or trimers. However, in our AUC experiments, FoxASD was present as a monomer (Fig 2C). This difference could be related to the presence of an N-terminal His-tag in the FoxASD protein used in the in vitro experiments, which was not present in the construct we used for the experiments in vivo. As such, it might be possible that the N-terminal His-tag inhibits the dimerization of FoxASD. Another possibility would be the formation of a heterodimer between FoxASD and FoxRC similarly to the secretin complexes. Moreover, it has been shown that in the periplasm SNT domains can interact with cytoplasmic membrane proteins through high homology regions (HR) located next to C-terminal PDZ domains within the cytoplasmic membrane proteins [35,36]. Interestingly, PDZ domains are present in several proteases, including the periplasmic proteases Prc and CtpA involved in activating CSS pathways [23,37]. Hence, one could suggest that the SD of TBDTs not only interact with the periplasmic domain of the anti-σ factor but also with proteases. In the same way, the SNT-like domain of the anti-σ factor could interact with proteases. In this regard, it is important to mention that, while the absence of Prc in a null mutant diminishes but not abolishes CSS activation, a proteolytically inactivated version of this protease exhibited a dominant negative effect and completely inhibited CSS activation [23]. This is likely the result of tight binding, but not cleavage, of Prc to the anti-σ factor or to another component of the CSS system, which agrees with the formation of a FoxRC/FoxASD/Prc complex. Additionally, the region downstream of the SD of FoxA interacts with TonB [10], an interaction that is required both for transporting the siderophore and for signaling. So, one might imagine the formation of different complexes in the periplasm regulating signaling and the transport of the siderophore through TBDTs.
Formation of an anti-σ factor/SD complex in the absence of the inducing signal has also been proposed by Jensen and colleagues after solving the structure of the PupR/PupBSD complex [16]. Because our attempts to obtain crystals of the FoxR/FoxASD complex were unsuccessful, we generated a structural prediction of this complex (Fig 3A). Most of the interactions detected in the FoxR/FoxA complex mirror those observed in the crystal structure of the PupR/PupB complex [16], which involve the STN-like domains of both proteins. By changing residues involved in the FoxR/FoxA interaction, we showed that disrupting any of the β strands conforming the β-sheet interface leads to a defective interaction between both proteins. However, disrupting the 3 hydrogen bonds stablished by the FoxA-S81 residue with FoxR-Y295 and -T310 did not impair the formation of the FoxR/FoxA complex (Fig 3C, FoxASD-S81A). Importantly, while the interacting FoxASD-S81A variant can block the proteolysis FoxR, the non-interacting FoxASD-S81P protein cannot (Fig 4A and 4B). This indicates that the interaction with FoxR is essential for FoxASD to be able to protect the anti-σ factor from proteolysis, which further suggests that FoxR and FoxA already interact in the absence of the inducing signal.
A constitutive complex in the absence of ferrioxamine is also formed between the FoxA receptor and the TonB protein [10]. This constitutive FoxA/TonB interaction is mediated by an unstructured polypeptide segment located upstream the TonB box of FoxA (residues 135–141). This segment is only present in TonB-dependent receptors involved in signaling which contain the additional N-terminal domain that forms the SD. Although the biological function of the TonB/FoxA constitutive interaction is still unknown, it has been proposed that it may be related to the signaling function of FoxA [10]. Binding of ferrioxamine to FoxA triggers conformational changes in extracellular loops of the protein but also in the periplasmic region provoking the expulsion of the TonB box from inside the barrel. This allows the formation of a high-affinity FoxA/TonB complex by β-augmentation, an interaction that is strengthened by complementary contacts between the unstructured polypeptide segment of FoxA and the surface of TonB [10]. Formation of this high-affinity FoxA/TonB complex triggers the signaling domain of FoxA to rotate but does not alter the overall structure of this domain [10]. Importantly, this displacement does not imply that FoxASD is further extended into the periplasm, as observed for other TBDTs [20]. Therefore, it is unclear how FoxASD can span that far across the periplasm to interact with FoxRC and link the cytoplasmic and the outer membranes. The solution to this problem may be related to our finding that FoxASD is cleaved, as we propose based on the detection of 2 different FoxA forms (Fig 4C, Δprc strain). Based on our previous results, we believe that the protease performing this cleavage is CtpA. We showed earlier that CtpA is required for proper CSS activity, and that this protease does not process the FoxR anti-σ factor but another component of the CSS pathway [23]. The phenotype of a ctpA mutant is in accordance with FoxASD not being cleaved because the FoxRC domain is very unstable in this mutant and CSS activity is considerably higher than in the wild-type strain ([23], see Figs 3B and 2A of this article). Moreover, we know that CtpA works upstream of Prc in the signaling cascade because a double prc ctpA mutant has the same phenotype as the single prc mutant [23]. Taking all these results into account, we propose that the SD of FoxA is cleaved, and that this cleavage is performed by the CtpA protease. We believe that, in the wild-type strain, the cleavage of FoxASD occurs in the absence of ferrioxamine, resulting in the stabilization of the FoxRC domain, which in turn blocks the RIP of the FoxRN domain and thus the release of σFoxI (S1B Fig). However, due to low amounts of the FoxA protein in the absence of ferrioxamine (Fig 4C), it has not been possible to detect this proteolytic event in the wild-type strain and further analysis is required to confirm this prediction. In the presence of ferrioxamine, rotation of the signaling domain of FoxA [10] would prevent the cleavage and release of FoxASD thus allowing the access of Prc to FoxRC. However, if the Prc protease is absent, the cleavage of FoxASD can occur in this condition (Fig 4C, Δprc). It is possible that Prc modulates CtpA levels or the access of CtpA to its substrate. Further experiments will be conducted to clarify this. Nevertheless, cleavage of the SD seems to be related to the signaling activity of TBDTs. In this context, it is important to note that, although signaling and transport are both substrate- and TonB-dependent processes, these functions are separable, and the SD of the TBDT can be removed without affecting transport [38] and transport can be blocked still allowing signaling [39]. Therefore, separation of FoxASD from the rest of the protein would still allow binding and transport of ferrioxamine.
In summary, we show in this work that FoxA and FoxR interact via their SNT-like domains, and that this interaction is required to protect the anti-σ factor from proteolysis. Furthermore, we propose that signal transduction via CSS involves the cleavage of the SD of FoxA, probably by the CtpA protease, since lack of this enzyme results in full-size FoxA protein and destabilization of FoxR. This evidence supports a novel model for CSS signaling in which in the absence of the signal, the SD of the TBDT is cleaved and protects the anti-σ from degradation under non-inducing conditions, providing an explanation for how these 2 proteins cover the physical gap between the cytoplasmic and outer membrane in the signal transduction pathway.
Materials and methods
Bacterial growth conditions
Bacteria were routinely grown in liquid LB [40] on a rotatory shaker at 37°C (P. aeruginosa and E. coli) or 30°C (P. putida) and 200 rpm. For iron-restricted conditions cells were cultured in CAS medium [27] containing 400 μm (for P. aeruginosa) or 200 μm (for P. putida) of 2,2′-bipyridyl. For induction experiments, the iron-restricted medium was supplemented with 1 μm iron-free ferrioxamine B (Merck), 40 μm iron-free ferrichrome (Santa Cruz Biotechnology), 20 μm haem (Merck), or aerobactin-containing supernatant in 1:1 proportion as described before [21]. Iron-rich conditions were obtained by adding 50 to 100 μm FeCl3 to the iron-restricted medium. When required, 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to the medium to induce full expression from the pMMB67EH Ptac promoter. Antibiotics were used at the following final concentrations (μg ml-1): ampicillin (Ap), 100; gentamycin (Gm), 10; kanamycin (Km), 50; piperacillin (Pip), 25; streptomycin (Sm), 100; tetracycline (Tc), 12.5 for E. coli and 20 for Pseudomonas.
Plasmid construction and molecular biology
Plasmids used are described in S1 Table and primers listed in S2 Table. PCR amplifications were performed using Phusion Hot Start High-Fidelity DNA Polymerase (Finnzymes) or Expand High Fidelity DNA polymerase (Roche). All constructs were confirmed by DNA sequencing and transferred to Pseudomonas by electroporation [41].
Bacterial strains and mutant construction
Strains used are listed in S1 Table. The P. aeruginosa ΔfoxR deletion mutant was constructed by allelic exchange using the suicide vector pKNG101 [42]. Briefly, a 500-bp DNA fragment of the 5′ (up) and 3′ (down) ends of the foxR gene were obtained by PCR using PAO1 chromosomal DNA as a template with proper oligonucleotides (S2 Table). The gene fragment containing the foxR deletion was cloned into pKNG101 (S1 Table). The pKNG101-derivatives were maintained in E. coli CC118λpir and mobilized into P. aeruginosa PAO1 by triparental mating using the E. coli 1047 (pRK2013) helper strain [43]. Clones in which a double recombination event occurred after counterselection on sucrose plates were selected. The foxR chromosomal deletion was verified by PCR.
Enzyme assay
β-galactosidase activities in soluble cell extracts were determined using o-nitrophenyl-b-D-galactopyranoside (ONPG) (Merck) as described before [27]. Each condition was tested in duplicate in at least 3 biologically independent experiments and the data given are the average with error bars representing standard deviation (SD). Activity is expressed in Miller units.
Protein purification
His-tagged proteins were produced in E. coli BL21 from pET28(+)-derivative plasmids and purified by affinity chromatography. Cells were grown in 2 L Erlenmeyer flasks containing 500 ml LB medium supplemented with 50 μg ml−1 kanamycin at 30°C until an OD660 of 0.6, at which point protein production was induced by adding 0.1 mM IPTG. Growth was continued at 18°C overnight prior to cell harvest by centrifugation at 10.000 × g for 30 min. Cell pellets were resuspended in 30 ml of buffer A (20 mM Tris-HCl, 0.1 mM EDTA, 300 mM NaCl, 5% (v/v) glycerol, 10 mM imidazole, 5 mM β-mercaptoethanol; pH 8.3) supplemented with 1× Complete protease inhibitor cocktail (Roche) and broken by sonication 5 times for 45 s. Following centrifugation at 20.000 × g, 4°C for 1 h the soluble fraction was passed through a 0.22 μm filter (Millipore) and loaded onto a 5 ml HisTrapHP chelating column (GE Healthcare) previously equilibrated in buffer A. Proteins were eluted with a 10 mM to 500 mM imidazole gradient in buffer A and dialyzed against buffer B (50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1 mM DTT).
Production of FoxR and FoxA antibodies
FoxRperi-T192A was purified as described above and sent to Innovagen (Sweden) for antibody production. Rabbits were immunized at day 0 and subsequently given boosters at days 14, 28, 49, and 70. At day 84, rabbits were killed and serum was isolated. Prior western blot, serum was concentrated using 30K centrifugal filter units (Millipore) at 4,000 rpm for 15 min. The FoxA antibody was produced by immunizing rabbits with a synthetic 14-amino acid peptide SDTQFDHVKEERYA (amino acids 331–344) in Abyntek (Spain). This peptide was designed from a predicted highly antigenic sequence of the protein. To increase antigenicity, the peptide was coupled to the carrier protein keyhole limpet hemocyanin (KLH). To remove nonspecific antibodies, the final antibody preparation was purified by affinity chromatography with the synthetic peptide used in the immunization protocol.
SDS-PAGE and western blot
Bacteria were grown until late log phase and pelleted by centrifugation. Samples were normalized according to the OD660 of the culture, solubilized in Laemmli buffer and heated for 10 min at 95°C. Proteins were separated by SDS-PAGE containing 12% or 15% (w/v) acrylamide and electrotransferred to nitrocellulose membranes. Ponceau S staining was performed as a loading control. Immunodetection was realized using a monoclonal antibody directed against the influenza hemagglutinin epitope (1:1,000) (HA.11, Covance) or polyclonal antibodies directed against FoxRperi (1:500) and FoxA (1:1,000). Detection of the OprL outer membrane lipoprotein with the monoclonal MA1-6 antibody [44] was used as an extra loading control. The second antibody, either the horseradish peroxidase-conjugated rabbit anti-mouse (DAKO) or the horseradish peroxidase-conjugated goat anti-rabbit IgG (Merck), was detected using SuperSignal West Chemiluminescent Substrates (Thermo Scientific). Blots were scanned and analyzed using the Quantity One version 4.6.7 (Bio-Rad).
Analytical ultracentrifugation
Purified proteins were dialysed against a buffer containing 20 mM Tris, 10% glycerol (vol/vol), 500 mM NaCl, 2 mM β-mercaptoethanol, pH 8.3, and injected onto sepharose column and subjected to size exclusion chromatography. Harvested fractions were dialysed against AUC buffer (20 mM Tris, 3% glycerol (vol/vol), 500 mM NaCl, 1 mM DTT, pH 8.3) and normalized to indicated protein concentrations. Experiments were performed with a Beckman Coulter Proteomelab XL-I analytical ultracentrifuge equipped with a Ti-50 rotor and double-sector cells. The dialysis buffer was used as reference in the cell. The rotor speed was set to 48,000 rpm. Rayleigh interference optics and absorbance optics at λ = 228 nm were used to monitor the sedimentation profiles. The temperature was set to 8°C. Data were collected at time intervals of 1 min for a total duration of the assays of 15 h. Scans were analyzed using Sedfit software (version 16.1c) [45]. Sedimentation coefficient (s-value) distributions were obtained from sedimentation velocity experiments using the c(s) method with Simplex algorithm fitting and Tikhonov-Philips regularization options. Density and viscosity of the solvent, and partial specific volume of the proteins (vbar) were calculated via Sednterp [46]. Sedimentation coefficient values given in the text were standardized to water and 20°C conditions using the calculated viscosity and density of the solvent at the conditions of the experiment. After fitting, data were exported to GUSSI v. 1.3.2 [47] for the preparation of figures. Hydrodynamic models, for the calculation of the theoretical sedimentation coefficient of the different oligomeric species (monomers and hetero-dimer), were generated using the software HHYDROPRO [32] with AlphaFold2 [33] as the source of the structural models with a mean confidence of over 80% for the best one. Best fit to isotherms of weight-average sedimentation coefficients was done by SEDPHAT version 15-2b [34], from a global analysis of both absorbance and interference data.
Computer-assisted analyses
Sequence analyses of the Pseudomonas genomes were performed at http://www.pseudomonas.com [48] and sequence alignments with ClustalW [49]. Protein tertiary structure and interaction models were obtained using Alphafold2 and Colabfold servers [33,50] and visualized using PyMOL (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC). The statistical analyses described in this work were conducted using Prism 7.0 software (GraphPad).
Supporting information
S1 Fig. The P. aeruginosa Fox CSS system.
(A) Structure of the FoxA TBDT. The 22-stranded antiparallel β-barrel domain of FoxA is shown in blue and the plug domain occluding the pore in yellow. The signaling domain (SD) is shown in red and the TonB box in orange. Structure was solved in [10] and was downloaded from Protein Data Bank (PDB code 6I97). (B) Schematic representation of the Fox system. The 3 components of the CSS system—receptor (FoxA), anti-σ factor (FoxR), and σECF (σFoxI)—are shown as well as the TonB-ExbBD complex and the proteases involved in the proteolysis of FoxR. Upon synthesis, the P. aeruginosa anti-σ factor FoxR undergoes a spontaneous cleavage that produces 2 functional N- and C-domains (FoxRN and FoxRC) that interact with each other in the periplasm and are both required for proper function. The FoxA receptor interacts with the TonB protein, which enables the energy coupled uptake of the siderophore ferrioxamine, and with the FoxRC domain via its signaling domain (FoxASD, red ball). In response to ferrioxamine, FoxRC is degraded by the C-terminal periplasmic protease Prc, and this event triggers the regulated intramembrane proteolysis (RIP) of the FoxRN domain by the action of (at least) 2 proteases: a (still unidentified) site-1 protease (S1P) and the site-2 RseP protease (S2P). This results in the release of σFoxI into the cytoplasm bound to the anti-σ domain of FoxR (FoxRASD). Although not experimentally demonstrated yet, FoxRASD likely forms part of the transcription complex. Among other genes, σFoxI promotes the transcription of the foxA receptor gene. OM, outer membrane; CM, cytoplasmic membrane; RNAP, RNA polymerase.
https://doi.org/10.1371/journal.pbio.3002920.s001
(TIF)
S2 Fig. Effect of overproducing FoxASD on FoxR stability.
(A) Scheme of the P. aeruginosa FoxR protein. The cytosolic, transmembrane, and periplasmic regions of the protein are shown. The numbers indicate amino acid positions. The site at which the self-cleavage of FoxR occurs (between Gly-191 and Thr-192) is indicated. The N- and C-domains resulting from self-cleavage are illustrated. (B, C) Western blot analyses of the P. aeruginosa FoxR protein in cells overproducing FoxASD. The P. aeruginosa ΔfoxR mutant containing the pMMB67EH empty plasmid (-) or its derivate expressing FoxR, HA-FoxR, or FoxR-HA, and the pBBR1MCS-5 empty plasmid (-) or its derivative expressing FoxASD were grown in iron-restricted medium supplemented with 1 mM IPTG and 1 μm ferrioxamine B (B) or with 50 mM FeCl3 (C). Proteins were separated by SDS-PAGE and immunoblotted against FoxR using the FoxRperi polyclonal antibody (upper panel) and FoxA using the FoxASD polyclonal antibody (middle panel). Detection of the outer membrane lipoprotein OprL was used as loading control. Positions of the protein fragments and the molecular size marker (in kDa) are indicated. Presence of the HA-tag adds ∼1 kDa to the molar mass of the protein fragments. Blots are representatives of 3 biological replicates (N = 3). The raw data underlying the graphs shown in the figure can be found at Mendeley Data repository (Mendeley Data, V1, 10.17632/nxh4c8ymnn.2). Western blot can be found in S1 Raw Images.
https://doi.org/10.1371/journal.pbio.3002920.s002
(TIF)
S3 Fig. Detection of the P. aeruginosa FoxR protein by western blot and complementation of the P. aeruginosa ΔfoxR mutant.
(A) P. aeruginosa PAO1 wild-type strain bearing the pMMB67EH empty plasmid or its derivative expressing the FoxR protein were grown under iron-restricted conditions and 1 mM IPTG. Proteins were immunoblotted against the anti-σ factor FoxR using a polyclonal antibody. Positions of the protein fragments and the molecular size marker (in kDa) are indicated. (B) The P. aeruginosa PAO1 wild-type strain and its isogenic ΔfoxR mutant bearing the foxA::lacZ transcriptional fusion and the pMMB67EH empty (-) or the pMMB67EH-derived plasmid producing the FoxR, FoxR-HA, or HA-FoxR proteins (S1 Table) were grown under iron-limitation conditions and in absence (-) or presence (+) of 1 μm ferrioxamine B. Activity was determined by β-galactosidase assay and data are means ± SD from 3 biological replicates (N = 3). P-values were calculated by two-tailed t test by comparing the value obtained in the PAO1 wild-type strain with that of the mutant strains in the same growth condition and are represented in the graphs by *, P P P P 10.17632/nxh4c8ymnn.2). Western blot can be found in S1 Raw Images.
https://doi.org/10.1371/journal.pbio.3002920.s003
(TIF)
S4 Fig. Analysis of FoxR and FoxASD protein stability.
E. coli BL21 cells producing the indicated protein domain were grown as described in Materials and methods. Cultures were harvested, resuspended in buffer A at different pHs, and subjected to sonication. The gel shows protein samples from the soluble (S) and the pellet (P) fractions after cells were resuspended in buffer A at pH 8.0, while other trials with various pHs showed similar results. Position the molecular size marker (in kDa) is indicated. Western blot can be found in S1 Raw Images.
https://doi.org/10.1371/journal.pbio.3002920.s004
(TIF)
S5 Fig. Structural model of FoxR.
(A) The Alphafold protein structure of FoxR (AF_AFQ9I115F1) is shown. The different domains of the protein are indicated. (B, C) Structural alignment between the periplasmic portions of FoxR and PupR (6OVM) with focus on the 2 anti-parallel β strands of the STN domain (C).
https://doi.org/10.1371/journal.pbio.3002920.s005
(TIF)
S6 Fig. Structural homology between FoxRSTND and FoxASD.
Structural features of the FoxRSTND (A) and FoxASD (B) protein structures (obtained from AF_AFQ9I115F1 and 6I97, respectively). (C) Structural alignment between both protein domains.
https://doi.org/10.1371/journal.pbio.3002920.s006
(TIF)
S7 Fig. Structural prediction of the FoxRperi/FoxASD complex.
(A) Structural alignment between the Alphafold FoxRperi/FoxASD predicted complex (in blue and orange) and the PupB/PupR complex (in green, 6OVK) with an RSMD of 0.862. (B) Alphafold model for the FoxRperi/FoxASD complex colored by its pLDDT value. (C) PAE chart for the FoxRperi/FoxASD model showing a likely interaction between FoxASD and the FoxRperi proteins. The raw data underlying the graphs shown in the figure can be found at Mendeley Data repository (Mendeley Data, V1, 10.17632/nxh4c8ymnn.2).
https://doi.org/10.1371/journal.pbio.3002920.s007
(TIF)
Acknowledgments
We thank T. Krell for assistance with protein purification, and C. Civantos and A. Sánchez-Jiménez for technical assistance with western blot experiments.
References
- 1.
Noinaj N, Guillier M, Barnard TJ, Buchanan SK. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64:43–60. pmid:20420522; PubMed Central PMCID: PMC3108441. - 2.
Sánchez-Jiménez A, Marcos-Torres FJ, Llamas MA. Mechanisms of iron homeostasis in Pseudomonas aeruginosa and emerging therapeutics directed to disrupt this vital process. J Microbial Biotechnol. 2023;16(7):1475–1491. Epub 20230301. pmid:36857468; PubMed Central PMCID: PMC10281387. - 3.
Cornelis P, Matthijs S. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ Microbiol. 2002;4(12):787–798. 47. pmid:12534462 - 4.
Cornelis P, Bodilis J. A survey of TonB-dependent receptors in fluorescent pseudomonads. Environ Microbiol Rep. 2009;1(4):256–262. pmid:23765855. - 5.
Llamas MA, Imperi F, Visca P, Lamont IL. Cell-surface signaling in Pseudomonas: stress responses, iron transport, and pathogenicity. FEMS Microbiol Rev. 2014;38(4):569–597. pmid:24923658. - 6.
Otero-Asman JR, Wettstadt S, Bernal P, Llamas MA. Diversity of extracytoplasmic function sigma (σECF) factor-dependent signaling in Pseudomonas. Mol Microbiol. 2019;112(2):356–373. pmid:31206859. - 7.
Braun V, Hartmann MD, Hantke K. Transcription regulation of iron carrier transport genes by ECF sigma factors through signaling from the cell surface into the cytoplasm. FEMS Microbiol Rev. 2022;46(4). pmid:35138377; PubMed Central PMCID: PMC9249621. - 8.
Koebnik R. TonB-dependent trans-envelope signalling: the exception or the rule? Trends Microbiol. 2005;13(8):343–347. pmid:15993072. - 9.
Wirth C, Meyer-Klaucke W, Pattus F, Cobessi D. From the periplasmic signaling domain to the extracellular face of an outer membrane signal transducer of Pseudomonas aeruginosa: crystal structure of the ferric pyoverdine outer membrane receptor. J Mol Biol. 2007;368(2):398–406. 138. pmid:17349657 - 10.
Josts I, Veith K, Tidow H. Ternary structure of the outer membrane transporter FoxA with resolved signalling domain provides insights into TonB-mediated siderophore uptake. Elife. 2019;8. Epub 2019/08/07. pmid:31385808; PubMed Central PMCID: PMC6699858. - 11.
Garcia-Herrero A, Vogel HJ. Nuclear magnetic resonance solution structure of the periplasmic signalling domain of the TonB-dependent outer membrane transporter FecA from Escherichia coli. Mol Microbiol. 2005;58(5):1226–1237. 139. - 12.
Brillet K, Journet L, Celia H, Paulus L, Stahl A, Pattus F, et al. A beta strand lock exchange for signal transduction in TonB-dependent transducers on the basis of a common structural motif. Structure. 2007;15(11):1383–1391. 193. pmid:17997964 - 13.
Korotkov KV, Gonen T, Hol WG. Secretins: dynamic channels for protein transport across membranes. Trends Biochem Sci. 2011;36(8):433–443. Epub 20110511. pmid:21565514; PubMed Central PMCID: PMC3155655. - 14.
Barbat B, Douzi B, Voulhoux R. Structural lessons on bacterial secretins. Biochimie. 2023;205:110–116. Epub 20220909. pmid:36096236. - 15.
Jensen JL, Balbo A, Neau DB, Chakravarthy S, Zhao H, Sinha SC, et al. Mechanistic implications of the unique structural features and dimerization of the cytoplasmic domain of the Pseudomonas sigma regulator, PupR. Biochemistry. 2015;54(38):5867–5877. Epub 20150914. pmid:26313375; PubMed Central PMCID: PMC4701049. - 16.
Jensen JL, Jernberg BD, Sinha SC, Colbert CL. Structural basis of cell-surface signaling by a conserved sigma regulator in Gram-negative bacteria. J Biol Chem. 2020;295(17):5795–5806. Epub 2020/02/29. pmid:32107313; PubMed Central PMCID: PMC7186176. - 17.
Enz S, Brand H, Orellana C, Mahren S, Braun V. Sites of interaction between the FecA and FecR signal transduction proteins of ferric citrate transport in Escherichia coli K-12. J Bacteriol. 2003;185(13):3745–3752. 160. - 18.
Breidenstein E, Mahren S, Braun V. Residues involved in FecR binding are localized on one side of the FecA signaling domain in Escherichia coli. J Bacteriol. 2006;188(17):6440–6442. 217. - 19.
Bastiaansen KC, Civantos C, Bitter W, Llamas MA. New insights into the regulation of cell-surface signaling activity acquired from a mutagenesis screen of the Pseudomonas putida IutY sigma/anti-sigma factor. Front Microbiol. 2017;8-747(747):1–15. pmid:28512454; PubMed Central PMCID: PMC5411451. - 20.
Mokdad A, Herrick DZ, Kahn AK, Andrews E, Kim M, Cafiso DS. Ligand-induced structural changes in the Escherichia coli ferric citrate transporter reveal modes for regulating protein-protein interactions. J Mol Biol. 2012;423(5):818–830. Epub 20120911. pmid:22982293; PubMed Central PMCID: PMC3472153. - 21.
Bastiaansen KC, Ibañez A, Ramos JL, Bitter W, Llamas MA. The Prc and RseP proteases control bacterial cell-surface signalling activity. Environ Microbiol. 2014;16(8):2433–2443. Epub 2014/01/01. pmid:24373018. - 22.
Bastiaansen KC, Otero-Asman JR, Luirink J, Bitter W, Llamas MA. Processing of cell-surface signalling anti-sigma factors prior to signal recognition is a conserved autoproteolytic mechanism that produces two functional domains. Environ Microbiol. 2015;17(9):3263–3277. Epub 2015/01/13. pmid:25581349. - 23.
Otero-Asman JR, Sánchez-Jiménez A, Bastiaansen KC, Wettstadt S, Civantos C, García-Puente A, et al. The Prc and CtpA proteases modulate cell-surface signaling activity and virulence in Pseudomonas aeruginosa. iScience. 2023;26(7):107216. pmid:37534181 - 24.
Chan DCK, Josts I, Koteva K, Wright GD, Tidow H, Burrows LL. Interactions of TonB-dependent transporter FoxA with siderophores and antibiotics that affect binding, uptake, and signal transduction. Proc Natl Acad Sci U S A. 2023;120(16):e2221253120. Epub 20230412. pmid:37043535; PubMed Central PMCID: PMC10120069. - 25.
Chan DCK, Burrows LL. Thiocillin and micrococcin exploit the ferrioxamine receptor of Pseudomonas aeruginosa for uptake. J Antimicrob Chemother. 2021;76(8):2029–2039. pmid:33907816. - 26.
Normant V, Josts I, Kuhn L, Perraud Q, Fritsch S, Hammann P, et al. Nocardamine-dependent iron uptake in Pseudomonas aeruginosa: exclusive involvement of the FoxA outer membrane transporter. ACS Chem Biol. 2020;15(10):2741–2751. Epub 20200924. pmid:32902248. - 27.
Llamas MA, Sparrius M, Kloet R, Jimenez CR, Vandenbroucke-Grauls C, Bitter W. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J Bacteriol. 2006;188(5):1882–1891. 2. - 28.
Bastiaansen KC, van Ulsen P, Wijtmans M, Bitter W, Llamas MA. Self-cleavage of the Pseudomonas aeruginosa Cell-surface signaling anti-sigma factor FoxR occurs through an N-O acyl rearrangement. J Biol Chem. 2015;290(19):12237–12246. Epub 2015/03/27. pmid:25809487; PubMed Central PMCID: PMC4424355. - 29.
Otero-Asman JR, Garcia-Garcia AI, Civantos C, Quesada JM, Llamas MA. Pseudomonas aeruginosa possesses three distinct systems for sensing and using the host molecule haem. Environ Microbiol. 2019;21(12):4629–4647. pmid:31390127. - 30.
Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A. 1998;95(10):5752–5756. pmid:9576956; PubMed Central PMCID: PMC20451. - 31.
Ouellette SP, Karimova G, Davi M, Ladant D. Analysis of membrane protein interactions with a bacterial adenylate cyclase-based two-hybrid (BACTH) technique. Curr Protoc Mol Biol. 2017;118:20 12 1–20 12 24. pmid:28369675. - 32.
Ortega A, Amoros D, Garcia de la Torre J. Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophys J. 2011;101(4):892–898. pmid:21843480; PubMed Central PMCID: PMC3175065. - 33.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. Epub 20210715. pmid:34265844; PubMed Central PMCID: PMC8371605. - 34.
Schuck P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal Biochem. 2003;320(1):104–124. pmid:12895474. - 35.
Login FH, Fries M, Wang X, Pickersgill RW, Shevchik VE. A 20-residue peptide of the inner membrane protein OutC mediates interaction with two distinct sites of the outer membrane secretin OutD and is essential for the functional type II secretion system in Erwinia chrysanthemi. Mol Microbiol. 2010;76(4):944–955. Epub 2010/05/07. pmid:20444086. - 36.
Korotkov KV, Krumm B, Bagdasarian M, Hol WG. Structural and functional studies of EpsC, a crucial component of the type 2 secretion system from Vibrio cholerae. J Mol Biol. 2006;363(2):311–321. Epub 2006/09/19. pmid:16978643. - 37.
Wettstadt S, Llamas MA. Role of regulated proteolysis in the communication of bacteria with the environment. Front Mol Biosci. 2020;7:586497. pmid:33195433; PubMed Central PMCID: PMC7593790. - 38.
Kim I, Stiefel A, Plantor S, Angerer A, Braun V. Transcription induction of the ferric citrate transport genes via the N-terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol Microbiol. 1997; 23(2):333–44. Epub 1997/01/01. pmid:9044267. - 39.
James HE, Beare PA, Martin LW, Lamont IL. Mutational analysis of a bifunctional ferrisiderophore receptor and signal-transducing protein from Pseudomonas aeruginosa. J Bacteriol. 2005; 187(13):4514–20. Epub 2005/06/22. pmid:15968062; PubMed Central PMCID: PMCPMC1151750. - 40.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor, N.Y.; 1989. - 41.
Choi KH, Kumar A, Schweizer HP. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 2006;64(3):391–397. Epub 2005/07/01. pmid:15987659. - 42.
Kaniga K, Delor I, Cornelis GR. A wide-host-range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109(1):137–141. pmid:1756974. - 43.
Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979;76(4):1648–1652. PubMed Central PMCID: PMC383447. pmid:377280 - 44.
Hancock RE, Wieczorek AA, Mutharia LM, Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982;37(1):166–171. pmid:6809625; PubMed Central PMCID: PMC347506. - 45.
Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J. 2000;78(3):1606–1619. pmid:10692345; PubMed Central PMCID: PMC1300758. - 46.
Philo JS. SEDNTERP: a calculation and database utility to aid interpretation of analytical ultracentrifugation and light scattering data. Eur Biophys J. 2023;52(4–5):233–266. Epub 20230215. pmid:36792822. - 47.
Brautigam CA. Calculations and publication-quality illustrations for analytical ultracentrifugation data. Methods Enzymol. 2015;562:109–133. Epub 20150616. pmid:26412649. - 48.
Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, Yu NY, et al. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 2011;39:D596–D600. Epub 2010/10/12. pmid:20929876; PubMed Central PMCID: PMC3013766. - 49.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. pmid:7984417; PubMed Central PMCID: PMC308517. - 50.
Mirdita M, Schutze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. ColabFold: making protein folding accessible to all. Nat Methods. 2022;19(6):679–682. Epub 20220530. pmid:35637307; PubMed Central PMCID: PMC9184281.
ADVERTISEMENT:
Halo, para pencinta slots pernahkah mendengar istilah “raja slot? Kalau belum, siap-siap jatuh cinta dengan konsep ini. slot gacor adalah mesin slot yang selalu kasih kemenangan. Ya, mesin-mesin ini bisa disebut adalah jagoannya tuk bawa come back hasil. but, cemana sih
tekniknya jumpain slot gacor yang tepat? Santai Bro and Sis, kita bahas santai aja di tempat ini
Games terpercaya waktu ini satu-satunya di Indonesia hanya di pasti memberikan ROI terbesar
Daftar hanya di :
Informasi mengenai KING SLOT, Segera Daftar Bersama king selot terbaik dan terpercaya no satu di Indonesia. Boleh mendaftar melalui sini king slot serta memberikan hasil kembali yang paling tinggi saat sekarang ini hanyalah KING SLOT atau Raja slot paling gacor, gilak dan gaco saat sekarang di Indonesia melalui program return tinggi di kingselot serta pg king slot
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama kdwapp.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama jebswagstore.com
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama demoslotgacor.pro
slot demo gacor
slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot gacor
akun demo slot gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo gacor
akun slot demo gacor permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot pragmatic
akun demo slot pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo pragmatic
akun slot demo pragmatic permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun slot demo
akun slot demo permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
akun demo slot
akun demo slot permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
situs slot terbaru
situs slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
slot terbaru
slot terbaru permainan paling top dan garansi imbal balik hasil besar bersama situsslotterbaru.net
suara88 permainan paling top dan garansi imbal balik hasil besar bersama suara88.biz
sumo7777 permainan paling top dan garansi imbal balik hasil besar bersama sumo7777.com
supermoney888 permainan paling top dan garansi imbal balik hasil besar bersama supermoney888.biz
teratai88 permainan paling top dan garansi imbal balik hasil besar bersama teratai88.biz
thor88 permainan paling top dan garansi imbal balik hasil besar bersama thor88.biz
togelhk88 permainan paling top dan garansi imbal balik hasil besar bersama togelhk88.net
topjitu88 permainan paling top dan garansi imbal balik hasil besar bersama topjitu88.net
totosloto88 permainan paling top dan garansi imbal balik hasil besar bersama totosloto88.com
trisula888 permainan paling top dan garansi imbal balik hasil besar bersama trisula888.biz
udangbet88 permainan paling top dan garansi imbal balik hasil besar bersama udangbet88.net
via88 permainan paling top dan garansi imbal balik hasil besar bersama via88.biz
virusjp88 permainan paling top dan garansi imbal balik hasil besar bersama virusjp88.net
warga888 permainan paling top dan garansi imbal balik hasil besar bersama warga888.biz
waw88 permainan paling top dan garansi imbal balik hasil besar bersama waw88.biz
winjitu88 permainan paling top dan garansi imbal balik hasil besar bersama winjitu88.net
wisdom88 permainan paling top dan garansi imbal balik hasil besar bersama wisdom88.biz
wnitogel88 permainan paling top dan garansi imbal balik hasil besar bersama wnitogel88.com
yoyo888 permainan paling top dan garansi imbal balik hasil besar bersama yoyo888.biz
validtoto88 permainan paling top dan garansi imbal balik hasil besar bersama validtoto88.com
sule999 permainan paling top dan garansi imbal balik hasil besar bersama sule999.com
sule88 permainan paling top dan garansi imbal balik hasil besar bersama sule88.org
ss888bet permainan paling top dan garansi imbal balik hasil besar bersama ss888bet.com
sia77 permainan paling top dan garansi imbal balik hasil besar bersama sia77.info
seluang88 permainan paling top dan garansi imbal balik hasil besar bersama seluang88.com
satu88 permainan paling top dan garansi imbal balik hasil besar bersama satu88.biz
satu777 permainan paling top dan garansi imbal balik hasil besar bersama satu777.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.biz
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp88.asia
rp88 permainan paling top dan garansi imbal balik hasil besar bersama rp77.live
qiuqiu88 permainan paling top dan garansi imbal balik hasil besar bersama qiuqiu88.biz
pt88 permainan paling top dan garansi imbal balik hasil besar bersama pt88.org
pt77 permainan paling top dan garansi imbal balik hasil besar bersama pt77.info
produk88 permainan paling top dan garansi imbal balik hasil besar bersama produk88.asia
mt88 permainan paling top dan garansi imbal balik hasil besar bersama mt88.org
mt77 permainan paling top dan garansi imbal balik hasil besar bersama mt77.biz
menang66 permainan paling top dan garansi imbal balik hasil besar bersama menang66.biz
latobet888 permainan paling top dan garansi imbal balik hasil besar bersama latobet888.org
kedai96 permainan paling top dan garansi imbal balik hasil besar bersama kedai96.org
kedai188 permainan paling top dan garansi imbal balik hasil besar bersama kedai188.biz
ids88 permainan paling top dan garansi imbal balik hasil besar bersama ids88.biz
hp88 permainan paling top dan garansi imbal balik hasil besar bersama hp88.org
hp77 permainan paling top dan garansi imbal balik hasil besar bersama hp77.org
gm88 permainan paling top dan garansi imbal balik hasil besar bersama gm88.asia
gm77 permainan paling top dan garansi imbal balik hasil besar bersama gm77.net
final888 permainan paling top dan garansi imbal balik hasil besar bersama final888.org
duit88 permainan paling top dan garansi imbal balik hasil besar bersama duit88.asia
duit168 permainan paling top dan garansi imbal balik hasil besar bersama duit168.biz
divisi88 permainan paling top dan garansi imbal balik hasil besar bersama divisi88.org
dewi500 permainan paling top dan garansi imbal balik hasil besar bersama dewi500.biz
devil88 permainan paling top dan garansi imbal balik hasil besar bersama devil88.info
cuputoto88 permainan paling top dan garansi imbal balik hasil besar bersama cuputoto88.com
cukongbet88 permainan paling top dan garansi imbal balik hasil besar bersama cukongbet88.asia
bom888 permainan paling top dan garansi imbal balik hasil besar bersama bom888.biz
bintaro888 permainan paling top dan garansi imbal balik hasil besar bersama bintaro888.info
askasino88 permainan paling top dan garansi imbal balik hasil besar bersama askasino88.org
999aset permainan paling top dan garansi imbal balik hasil besar bersama 999aset.com
afb77 permainan paling top dan garansi imbal balik hasil besar bersama afb77.biz
aset99 permainan paling top dan garansi imbal balik hasil besar bersama aset99.biz
bendera77 permainan paling top dan garansi imbal balik hasil besar bersama bendera77.biz
bendera888 permainan paling top dan garansi imbal balik hasil besar bersama bendera888.com
coco88 permainan paling top dan garansi imbal balik hasil besar bersama coco88.org
cuma77 permainan paling top dan garansi imbal balik hasil besar bersama cuma77.biz
cuma88 permainan paling top dan garansi imbal balik hasil besar bersama cuma88.org
dwv88 permainan paling top dan garansi imbal balik hasil besar bersama dwv88.org
fafajp88 permainan paling top dan garansi imbal balik hasil besar bersama fafajp88.com
gemar88 permainan paling top dan garansi imbal balik hasil besar bersama gemar88.biz
gocap88 permainan paling top dan garansi imbal balik hasil besar bersama gocap88.info
gocaptoto permainan paling top dan garansi imbal balik hasil besar bersama gocaptoto.asia
hakabet88 permainan paling top dan garansi imbal balik hasil besar bersama hakabet88.com
hwtoto88 permainan paling top dan garansi imbal balik hasil besar bersama hwtoto88.org
ina77 permainan paling top dan garansi imbal balik hasil besar bersama ina77.biz
ina88 permainan paling top dan garansi imbal balik hasil besar bersama ina88.info
jingga8888 permainan paling top dan garansi imbal balik hasil besar bersama jingga8888.com
juragan777 permainan paling top dan garansi imbal balik hasil besar bersama juragan777.asia
kastil77 permainan paling top dan garansi imbal balik hasil besar bersama kastil77.info
kebo888 permainan paling top dan garansi imbal balik hasil besar bersama kebo888.biz
kkwin77 permainan paling top dan garansi imbal balik hasil besar bersama kkwin77.com
kokoslot88 permainan paling top dan garansi imbal balik hasil besar bersama kokoslot88.asia
luckydf88 permainan paling top dan garansi imbal balik hasil besar bersama luckydf88.org
microstar888 permainan paling top dan garansi imbal balik hasil besar bersama microstar888.biz
monperatoto88 permainan paling top dan garansi imbal balik hasil besar bersama monperatoto88.com
mpo1122 permainan paling top dan garansi imbal balik hasil besar bersama mpo1122.biz
mpo122 permainan paling top dan garansi imbal balik hasil besar bersama mpo122.biz
mpopelangi88 permainan paling top dan garansi imbal balik hasil besar bersama mpopelangi88.com
pamanslot88 permainan paling top dan garansi imbal balik hasil besar bersama pamanslot88.biz
panel88 permainan paling top dan garansi imbal balik hasil besar bersama panel88.org
paragon77 permainan paling top dan garansi imbal balik hasil besar bersama paragon77.biz
paragon888 permainan paling top dan garansi imbal balik hasil besar bersama paragon888.info
pion77 permainan paling top dan garansi imbal balik hasil besar bersama pion77.biz
prada88 permainan paling top dan garansi imbal balik hasil besar bersama prada88.asia
prada888 permainan paling top dan garansi imbal balik hasil besar bersama prada888.com
qqslot88slot permainan paling top dan garansi imbal balik hasil besar bersama qqslot88slot.com
rejekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rejekibet88.com
rezekibet88 permainan paling top dan garansi imbal balik hasil besar bersama rezekibet88.org
sensa77 permainan paling top dan garansi imbal balik hasil besar bersama sensa77.biz
sensa888 permainan paling top dan garansi imbal balik hasil besar bersama sensa888.biz
singajp88 permainan paling top dan garansi imbal balik hasil besar bersama singajp88.com
sr77 permainan paling top dan garansi imbal balik hasil besar bersama sr77.org
sr88 permainan paling top dan garansi imbal balik hasil besar bersama sr88.org
surya77 permainan paling top dan garansi imbal balik hasil besar bersama surya77.biz
surya88 permainan paling top dan garansi imbal balik hasil besar bersama surya88.asia
tajir77 permainan paling top dan garansi imbal balik hasil besar bersama tajir77.info
tajir88 permainan paling top dan garansi imbal balik hasil besar bersama tajir88.biz
toto122 permainan paling top dan garansi imbal balik hasil besar bersama toto122.com
toto123 permainan paling top dan garansi imbal balik hasil besar bersama toto123.biz
uangvip88 permainan paling top dan garansi imbal balik hasil besar bersama uangvip88.com
wajik77 permainan paling top dan garansi imbal balik hasil besar bersama wajik77.asia
777neko permainan paling top dan garansi imbal balik hasil besar bersama 777neko.org
88judi permainan paling top dan garansi imbal balik hasil besar bersama 88judi.net
99judi permainan paling top dan garansi imbal balik hasil besar bersama 99judi.org
abcslot88 permainan paling top dan garansi imbal balik hasil besar bersama abcslot88.asia